Process for preparation of bedaquiline and pharmaceutically acceptable salts thereof
A technology of methoxyquinoline and dimethylamino, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as inconvenient process and large production time, and achieve the effect of economical and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
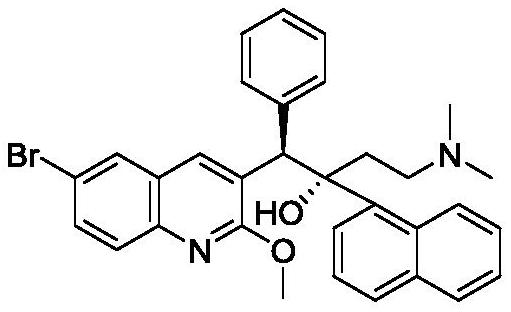
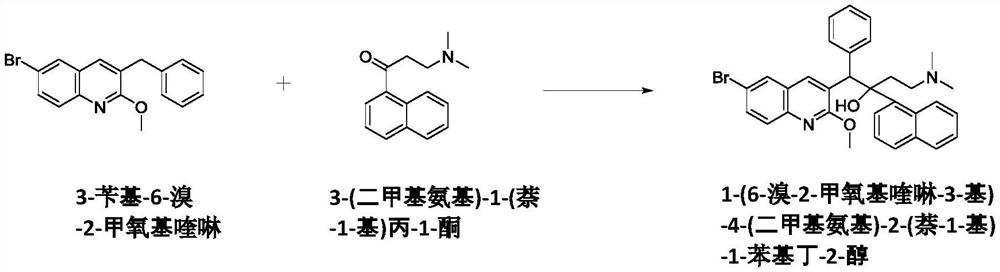
[0101] Preparation of 1-(6-bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2-(naphthalene-1-yl)-1-phenylbutyl Stereoisomeric mixture of -2-ol
[0102] Water (60 mL) was added to 3-(dimethylamino)-1'-propionylnaphthalene hydrochloride (50.6 g; 192 mmol), dissolved, diluted with toluene (500 mL), and stirred at room temperature to prepare a reaction Solution A. Sodium hydroxide (8.23 g; 206 mmol) was dissolved in water (60 mL), cooled to room temperature, added to the reaction solution A, and stirred for 30 minutes. The organic layer was separated therefrom, washed with water (60 mL), and dried over anhydrous sodium sulfate (25 g). The dried solution was filtered and concentrated under reduced pressure at an external temperature of 45°C. The concentrate was vacuum dried at room temperature for 2 hours to obtain product A.
[0103]Tetrahydrofuran (300 mL) was added to the nitrogen-filled first reaction portion followed by diethylamine (12.6 mL; 122 mmol) and N,N,N',N'-tetram...
Embodiment 2
[0104] From 1-(6-bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2-(naphthalene-1-yl)-1-phenylbutyl Separation of (1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-dimethylamino-2-(1-naphthalene from a stereoisomeric mixture of -2-ols Base)-1-phenyl-butan-2-ol
[0105] A. Preparation of (1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl- Butan-2-ol and salt of resolving agent
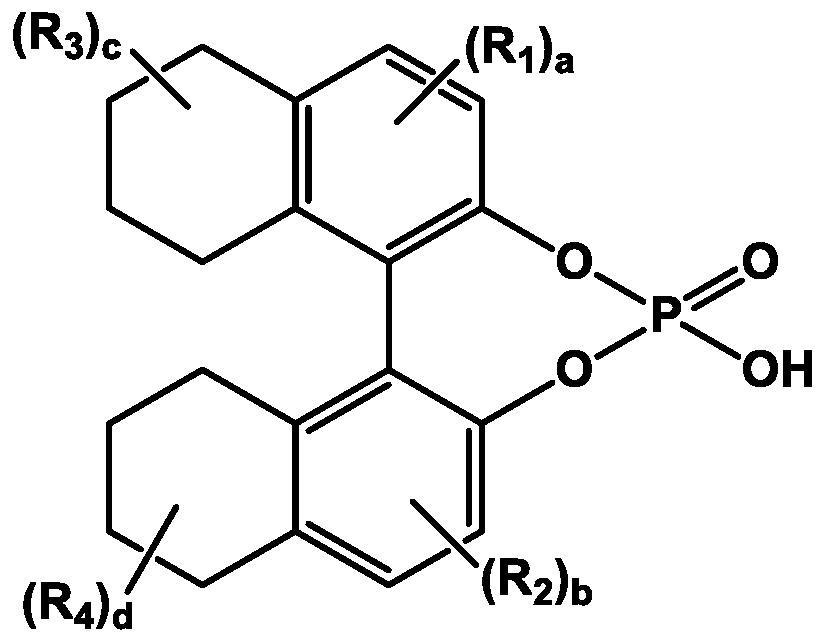
[0106] The powder obtained in Example 1 (2.8 g; 5.04 mmol) was added to the reaction portion, and then methanol (56 mL) and dimethylformamide (5.6 mL) were added and stirred. Addition of (11bR)-8,9,10,11,12,13,14,15-octahydro-4-hydroxy-4-oxide-binaphtho[2,1-d:1',2'-f ][1,3,2]dioxaphosphapine, heated to 80°C and stirred for 1 hour. Thereafter, the resulting mixture was cooled to room temperature, then cooled again to 0 to 5°C, and stirred for 90 minutes. The solid was filtered and washed with methanol (14 mL). The washed solid was suspended in methanol (21 mL) and dimethy...
Embodiment 3
[0109] Preparation of (1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-dimethylamino-2-(1-naphthyl)-1- Phenyl-butan-2-ol fumarate
[0110] The solid obtained in Step B of Example 2 (0.75 g; 1.35 mmol) was added to the reaction portion, followed by isopropanol (15 mL) and stirred. After addition of fumaric acid (0.17 g; 1.43 mmol), the resulting solution was stirred at reflux for 1 hour. The mixture was filtered and washed with isopropanol (3.8 mL). The mixture was cooled to 0 to 5°C and stirred for 2 hours. The solid was filtered and washed with isopropanol (3.8 mL). The resulting product was dried under vacuum at 30°C to obtain 0.78 g of (1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-dimethylamino-2- (1-Naphthyl)-1-phenyl-butan-2-ol fumarate. Yield: 86%, purity: 99.9%, optical purity: 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com