Bispecific antibodies and uses thereof
A bispecific antibody and antibody technology, applied in the field of protein engineering, can solve the problems of affecting affinity, producing immunogenicity, and insufficient production of bispecific antibodies effectively, so as to achieve improved binding ability, strong activation ability, and good binding active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0335] Example 1 The CH1 / CL binding interface can be used for the evaluation of modified amino acid sites
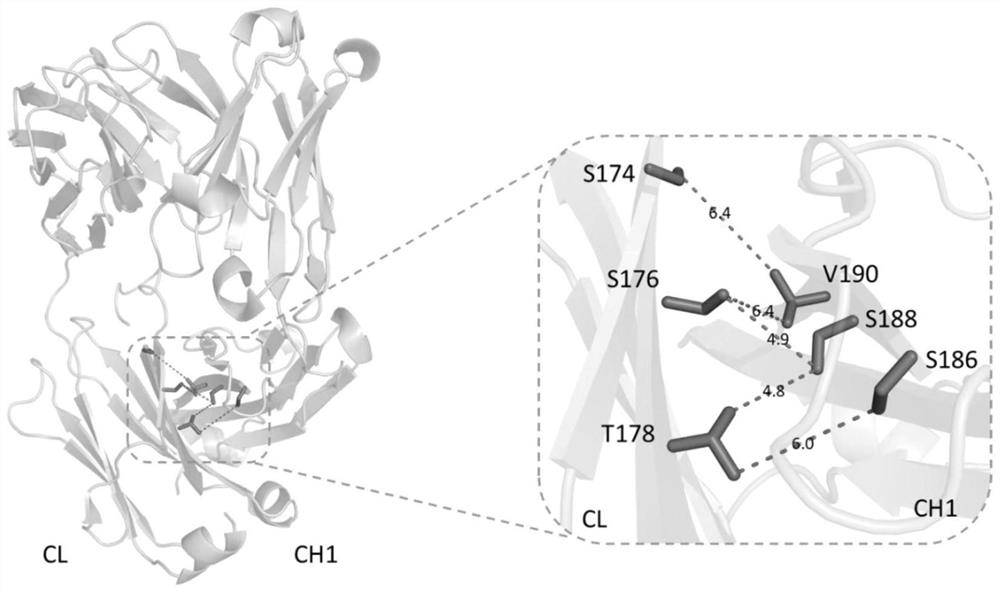
[0336] The amino acids at the CH1 / CL binding interface of the Fab constant region of the antibody maintain the interaction between the side chains through hydrophobic interactions, charge interactions, hydrogen bonds, etc. Inside. In this example, by analyzing the crystal structure of the CH1 / CL binding interface protein, the potential amino acid sites in the CH1 and CL domains of the bispecific antibody that can be used for engineering are obtained. The protein crystal structure was analyzed by PyMOL software (PDB ID: 3EO9), and the binding interface of E-strand in the CH1-CL domain can be found in figure 1 . Among them, the amino acid positions with strong interactions have been summarized in Table 1 (Summary of Modified Sites).
[0337] In the present invention, unless otherwise specified, amino acid numbers are described according to Kabat numbering. In this exa...
Embodiment 2
[0340] Example 2 Screening of amino acid site mutations at the CH1 / CL binding interface
[0341] In order to screen different CH1 / CL interfaces that can produce specific binding, the following methods are used to screen for mutation combinations with better CH1 / CL interface pairing effects:
[0342] Referring to the method of Hust et al. (Methods in Molecular Biology-Phage Display Volume 1701||Construction of Synthetic Antibody Phage-Display Libraries[J].2018.), the CH1-kCL binding interface in Table 1 can be used for the modified amino acid sites , design primers for random mutation of amino acids, and construct a Fab display library for random mutation of amino acid sites on the phage CH1 / CL interface.
[0343] After releasing, packaging, and precipitating the phage library, after three rounds of screening with the corresponding biotinylated antigen, the clones obtained from the screening were further detected by enzyme-linked immunosorbent assay, and the absorbance value (O...
Embodiment 3 4
[0352] Example 3 Design of four-chain bispecific antibody and construction of expression vector
[0353] (1) Design of four-chain bispecific antibody
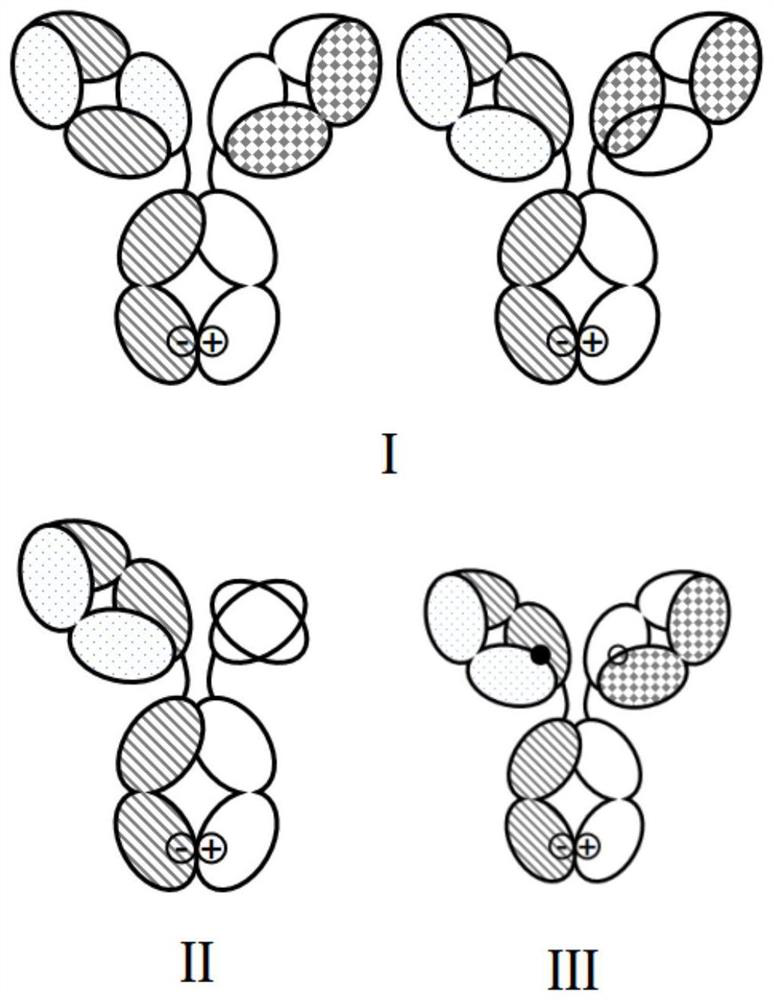
[0354] The exemplary bispecific antibody designed and constructed in this example is a "Y"-shaped IgG antibody (see figure 2 ), which includes 2 complete heterologous heavy chains and 2 complete heterologous light chains, which together form the Fab and Fc domains of the antibody.
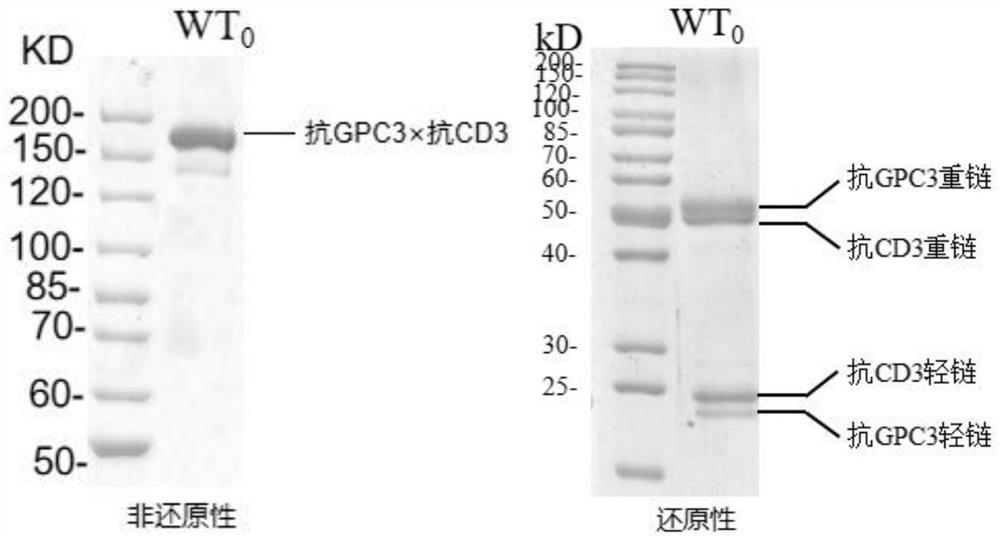
[0355] The target combination selected in this embodiment is GPC3 and CD3, but not limited to this target combination. The first Fab region of the bispecific antibody in this example binds to human CD3, and the second Fab region binds to human GPC3. The part specifically binding to human CD3 has a first heavy chain and a first light chain, and the part specifically binding to human GPC3 has a second heavy chain and a second light chain, and its sequence is derived from the GPC3 sequence of our company. The Fc part of the antibody is modified acc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap