Compositions and methods for improving outcome of treatment in patients with hematological malignancies using expanded stem cell products
A technology of stem cells and patients, applied in the direction of extracellular fluid diseases, biochemical equipment and methods, blood diseases, etc., can solve problems such as long-lasting remission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Preparation of Expanded Stem Cell Products
[0033] Expanded stem cell products comprise hematopoietic stem cells or hematopoietic stem cells and progenitors, of which T cells and red blood cells have been substantially depleted, and thus generally contain enriched numbers of CD34+ hematopoietic stem cells or hematopoietic stem cells and progenitors. Hematopoietic stem cells or hematopoietic stem and progenitor cells contain multiple HLA types because hematopoietic stem cells or hematopoietic stem and progenitor cells are mismatched with each other and with the patient before they are combined. As used herein, substantially depleted of T cells refers to less than 1% CD3+ cells, or less than 0.5% CD3+ cells, or less than 0.1% CD3+ cells in the expanded stem cell product.
[0034] In some embodiments, the CD34+ hematopoietic stem cells or hematopoietic stem and progenitor cells are derived from cord blood or placental blood. Human cord blood and / or human placental bloo...
Embodiment 1
[0121] Example 1: Generation of Human Expanded Stem Cell Products from Human Cord Blood Units
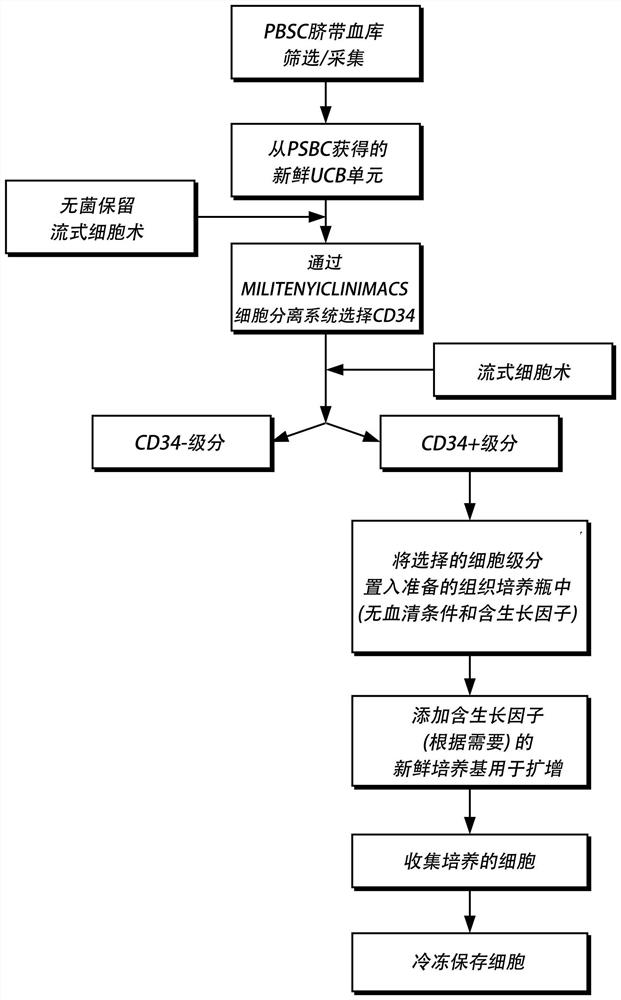
[0122] The following sections describe the production and storage of expanded stem cell products as figure 1 shown in the flow chart.
[0123] Cord blood / placental blood units are collected from human donors at birth. The collected blood is then mixed with an anticoagulant to prevent clotting. Blood was stored in isolation at 4°C in a monitored refrigerator. The received units are evaluated and the units to be processed for amplification are determined. The following information was collected on the unit: date of receipt, age of the unit in hours, gestational age of the donor in weeks, sex of the donor, and volume of the unit. In addition, determine the total nucleated cell count and total CD34+ cell count per unit and calculate the percentage of CD34+ cells. If the unit has fewer than 3.5 million CD34+ cells, the unit will be discarded. When a unit is selected for expansion,...
Embodiment 2
[0128] Example 2: Generation of Human Expanded Stem Cell Products from Frozen Human Cord Blood Units
[0129] An expanded stem cell product is prepared containing the total cell progeny generated from enriched CD34+ cells selected from pooled human cord blood units (pools of 4 to 20 individual units). As follows in the Notch ligand Delta1 ext-IgG Pooled human cord blood units were cultured in the presence of (DXI) and recombinant cytokines.
[0130]Option to use cord blood units with approximately 2 to 20 million cells. Cord blood units were thawed, centrifuged to remove cryoprotectants, resuspended in selection buffer and pooled into a single container. Selection buffer is usually PBS containing 1 mM EDTA and other components. Cord blood units are usually thawed in pairs. Cells were washed twice in the selected buffer. Cells were pooled without regard to HLA antigens or alleles (ie no matching required). Pooled cord blood units were pre-incubated with paramagnetic beads...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com