Raw material composition and method for preparing methionine hydroxyl analogue without byproduct salt separation by using raw material composition
A methionine hydroxyl and composition technology, which is applied in the preparation, application, animal feed and other directions of thioether, can solve the problems of high processing cost, complicated operation process, limited direct application range, etc., and achieves simple production process and strong controllability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 (1:1, MAP)
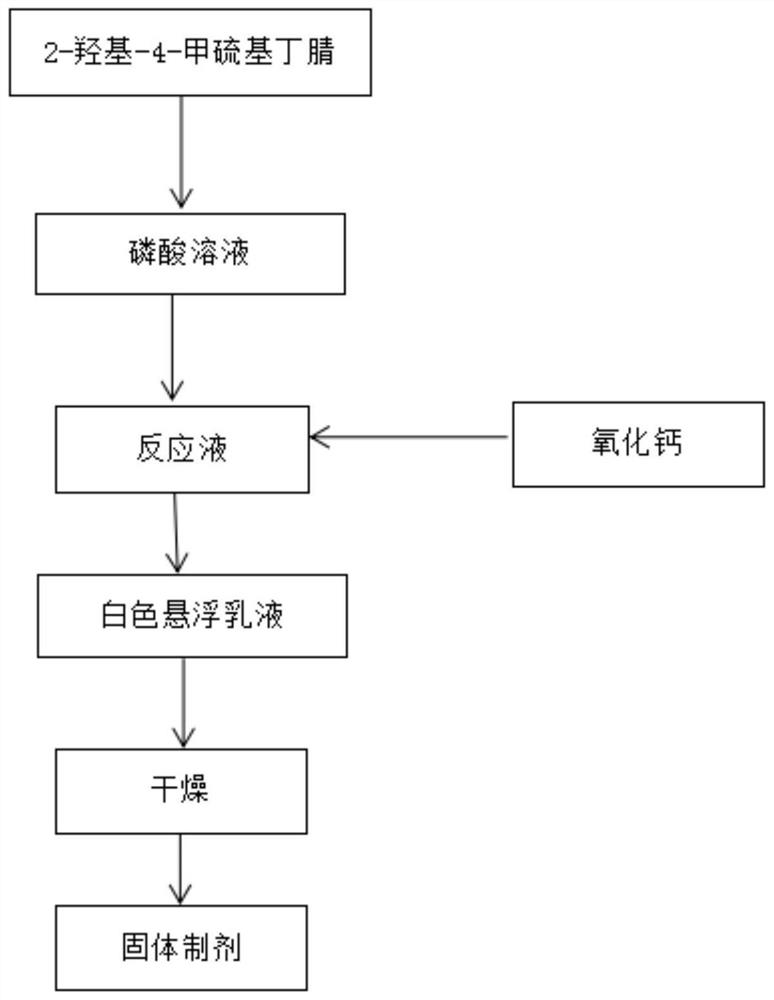
[0048] Install a 500ml four-necked flask with reflux condensation, a thermometer, and Teflon-lined stirring and place it in a water bath preheated to 50°C. Take 115.1g of phosphoric acid solution with a concentration of 85.22w% and transfer it to the above-mentioned four-necked flask. Start stirring, and add 154.8 g of stable stored 84.63% cyanohydrin feed solution (2-hydroxy-4-methylthiobutyronitrile, the rest is water) into the phosphoric acid solution in the above-mentioned four-necked flask, and heat it at 50° C. and stir for 30 minutes. Sampling is sent to HPLC, there is still a small amount of cyanohydrin residue, continue the insulation reaction for 30min, and monitor that the remaining cyanohydrin is completely converted into 2-hydroxyl-4-methylthiobutyramide (partially directly converted into 2-hydroxyl-4-methylthiobutyric acid ), add 40g of pure water under constant stirring, raise the temperature to 100°C and continue the reaction fo...
Embodiment 2
[0049] Embodiment 2 (1:1, MCP)
[0050] Install a 500ml four-necked flask with reflux condensation, a thermometer, and Teflon-lined stirring and place it in a water bath preheated to 50°C. Take 115.1g of phosphoric acid solution with a concentration of 85.22w% and transfer it to the above-mentioned four-necked flask. Start stirring, and add 154.8 g of stable stored 84.63% cyanohydrin feed solution (2-hydroxy-4-methylthiobutyronitrile, the rest is water) into the phosphoric acid solution in the above-mentioned four-necked flask, and heat it at 50° C. and stir for 30 minutes. Sampling is sent to HPLC, there is still a small amount of cyanohydrin residue, continue the insulation reaction for 30min, and monitor that the remaining cyanohydrin is completely converted into 2-hydroxyl-4-methylthiobutyramide (partially directly converted into 2-hydroxyl-4-methylthiobutyric acid ), add 60g of pure water under constant stirring, raise the temperature to 100°C and continue the reaction fo...
Embodiment 3
[0051] Example 3 (88% commercial grade MHA+phosphoric acid, calcium oxide, 1:1)
[0052] Transfer 170.5g of commercial grade 88% MHA into a 500mL beaker containing 115.1g of 85.22w% phosphoric acid solution, add 150ml of water to dilute the system until the water content is about 50%, keep stirring at 50°C for 1h, add 56.7g of calcium oxide (99 %) and 30ml of water configuration, stirred and reacted for 1h to obtain a slightly brown beige suspoemulsion, sprayed and dried using a small spray dryer to obtain a beige dispersible powdery solid with a more obvious MHA special smell, collected the solid at 105 ° C for two Drying for the first time to a constant weight yielded 293.50 g of beige tryptonine methionine hydroxy analog product, which had good dispersibility, and analyzed its content of methionine hydroxy analog: 49.62%, total phosphorus content 10.51%, water-soluble phosphorus content 8.98%, calcium content 13.63%, equivalent Methionine hydroxy analog calcium salt: 55.97%...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap