Aza-macrolide compound containing methyl guanidino urea as well as preparation method and application of aza-macrolide compound
A technology of macrocyclic compounds and compounds, applied in the field of azamacrolide compounds and their preparation, can solve the problems of high acquisition cost, incapable of large-scale application, difficult synthesis, etc., and achieve low acquisition cost and good insecticide Active, Synthetic Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
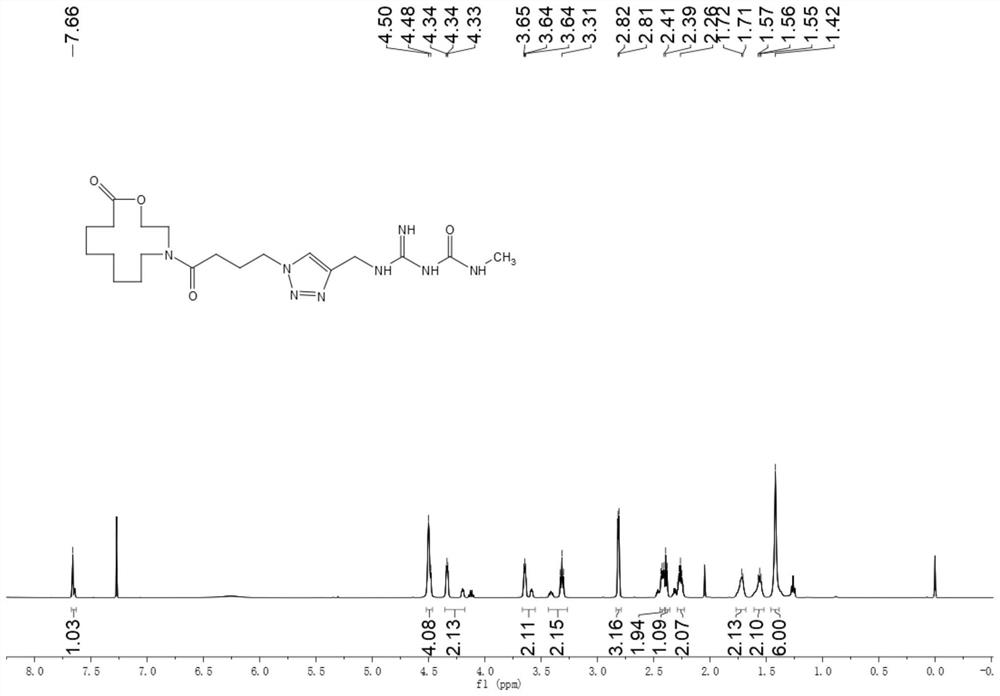
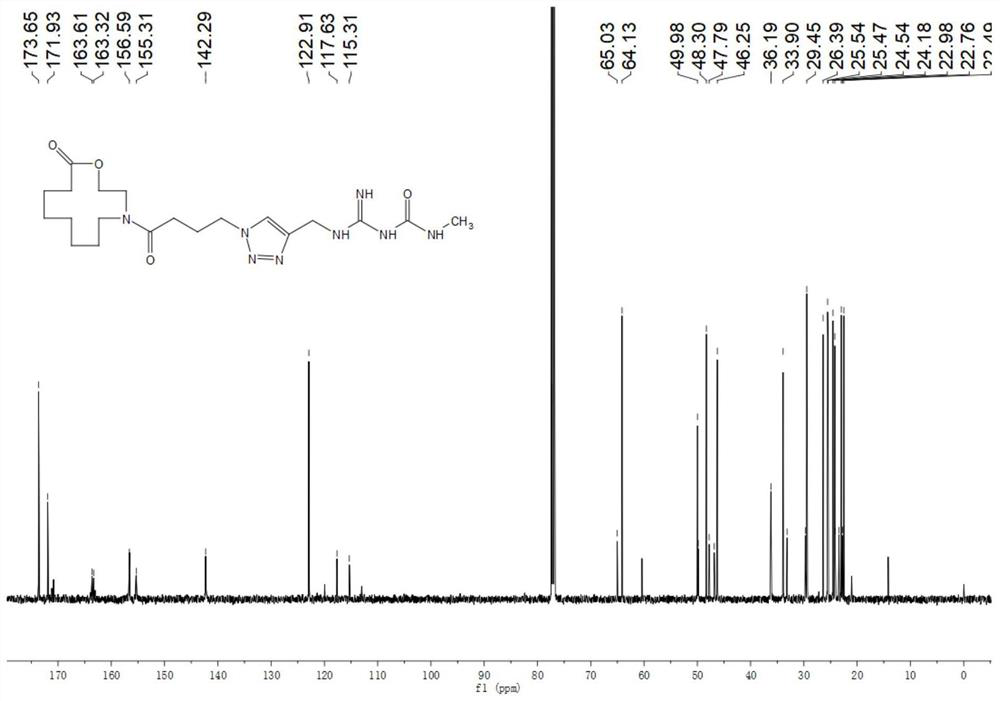
[0046] Embodiment 1, the preparation process of compound B6.
[0047]
[0048] Dissolve 0.01mol of bromoethanol and 0.03mol of sodium azide in 15ml of water, heat and stir at 100°C for 2 days, cool to room temperature after the reaction stops, extract the reaction solution with dichloromethane, collect the organic phase, and concentrate to obtain the product azide Nitroethanol was used directly in the next step without further purification.
[0049]
[0050]Dissolve 0.1 mol of cyclohexanone represented by formula IV and 0.1 mol of azide ethanol in 15 ml of dichloromethane, and slowly add 0.15 mol of boron trifluoride-diethyl ether solution dissolved in 5 ml of dichloromethane under stirring at room temperature into the reaction solution, and after the dropwise addition was completed, the mixture was stirred and reacted at 50° C. under reflux for 24 hours. After the reaction, use NaHCO 3 The reaction solution was extracted with an aqueous solution, the dichloromethane p...
Embodiment 2
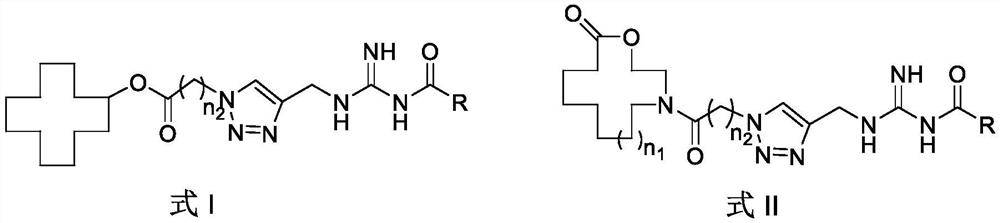
[0079] Embodiment 2, formula I and the compound enzyme inhibitory activity assay of formula II
[0080] Enzyme activity assay method: use 4-methylumbelliferyl N,N'-diacetyl-β-D-chitobioside (4-MethyluMbelliferyl N,N-diaacetyl-β-D-chitobioside) as the test substrate , chitinase (wherein the chitinase SmChiB of Serratia source is commercially purchased ( http: / / www.yingxinbio.com / ), the chitinase OfChi-h derived from Ostrinia cerevisiae was obtained according to the method reported by Professor Yang Qing et al. NaH 2 PO 4 , pH 6.8) were mixed in a 96-well plate to a final volume of 90 μL, 10 μL of 5 mM pNP-β-GlcNAc was added to initiate the reaction, incubated at 25 °C for 5 min, and 100 μL of 0.5 M sodium carbonate was added to terminate the reaction, and the absorbance was measured at 405 nm.
[0081] Compound inhibitory activity assay method: the sample was dissolved in DMSO and diluted into several different concentration gradients, ranging from 0.001-100 μM. On a 96-w...
Embodiment 3
[0084] Embodiment 3, general formula of the present invention is the determination of the insecticidal activity of the compound of formula I and formula II
[0085] Determination method: Treat the leaves with the dipping method, check the results after 3 days, lightly touch the insect body, and the individual that cannot crawl normally is regarded as dead, and the concentration of the test compound is 50mg / L. Calculate its adjusted mortality rate (%). Compared with the reference drugs diflubenzuron and hexaflumuron, the toxicity of the drug was judged. The insecticidal activity data of some compounds are shown in Table 4.
[0086] The following targets were tested:
[0087] Diamondback moth (Plutella xylostella Linnaeus), collected from vegetable fields in Beijing, was raised indoors with leaves of cruciferous vegetables at room temperature (27±1)°C, humidity 80%, light intensity 2000 lux, and light time of 1 day 12h. Under indoor rearing conditions, the 3rd instar larvae ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap