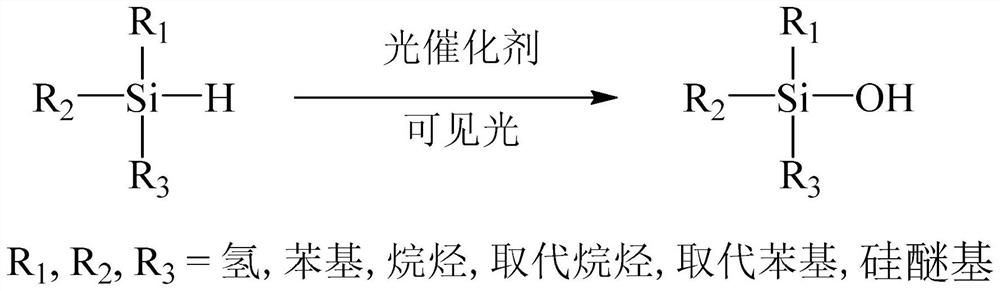
Method for preparing silanol by photocatalytic oxidation of silane
A technology for oxidizing silane and silanol, which is applied in the direction of silicon organic compounds, etc., can solve the problems of poor catalytic selectivity of precious metals, difficulty in preparing space silane, and unsuitability for industrial production, and achieve ultra-high activity and efficiency, high catalytic activity, and easy Separate reusable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] 1) Carbon nitride g-C 3 N 4 The preparation method:
[0042] A certain amount of urea or melamine is calcined in air at 550°C for 3-6h, the heating rate is 5°C / min, and after cooling to room temperature, a pale yellow powder is obtained. spare.
[0043] 2) Preparation of carbon quantum dots:
[0044] A certain amount of citric acid was pyrolyzed at 180°C with a heating rate of 5°C / min. After pyrolysis for 40 hours, 5 mol / L NaOH was added to neutralize the solution, and then the brown-orange powder was obtained by freeze-drying. spare.
[0045] 3) Bismuth Vanadate BiVO 4 Doping state preparation method:
[0046] At a certain amount of BiVO 4 Add Pd, Cu, Ru, Ir, Re, Ag, Pt, Au or Rh element or its compound state with a mass fraction of 0.01%-1%, calcined in air at 400°C for 2 hours, and the heating rate is 5°C / min.
[0047] 4) Titanium dioxide TiO 2 Doping state preparation method:
[0048] First, add a certain amount of Pd, Cu, Ru, Ir, Re, Ag, Pt, Au or Rh elem...
Embodiment 1
[0049] Embodiment 1, the preparation of compound dimethylphenylsilanol
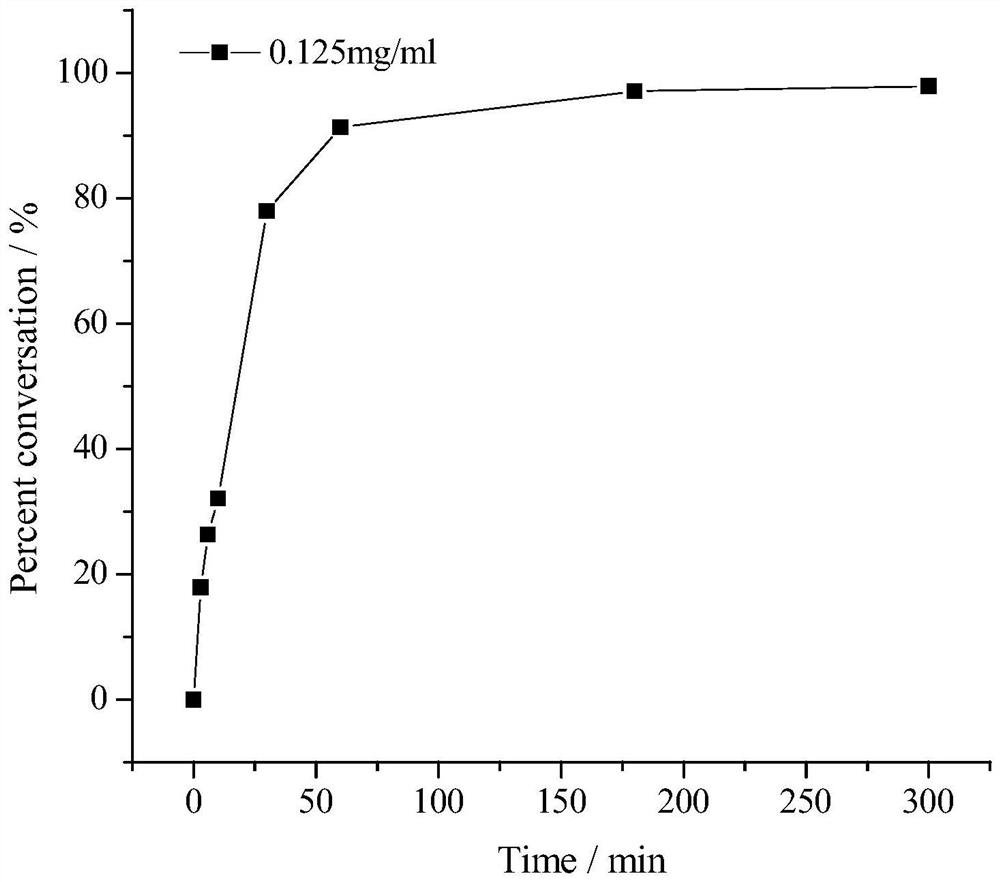
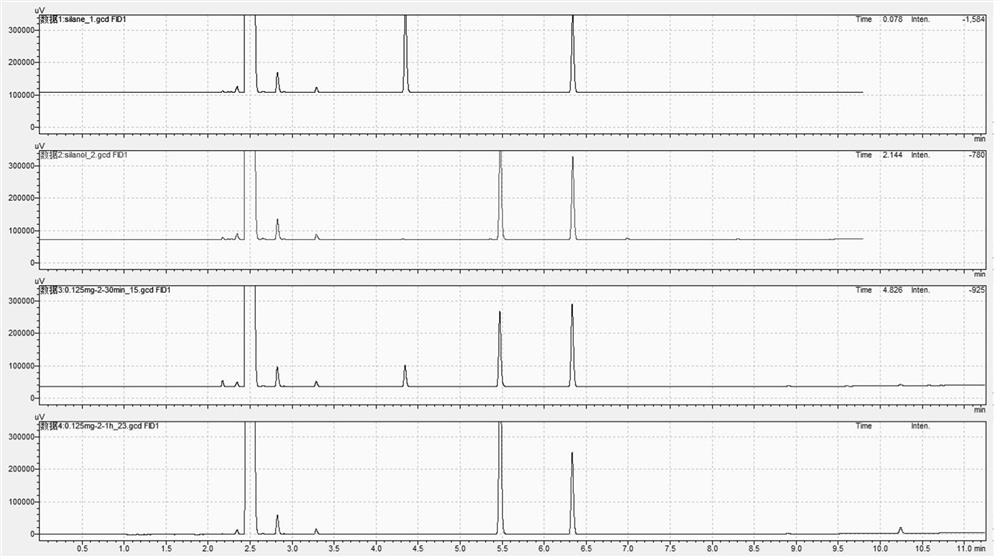
[0050] In a 10mL reaction flask, add 5mL water, add 50mM dimethylphenylsilane and 0.125mg Au-TiO 2 Under the induction of visible light, stir magnetically at room temperature for 30 minh. After the reaction, the catalyst was recovered by centrifugation, added 10ml of ethyl acetate for extraction, extracted twice, the organic phase was combined, the organic phase was distilled under reduced pressure at 30-40°C to remove the organic solvent, and the target compound dimethylbenzene was obtained by column chromatography elution Silanols (99% conversion, 98% selectivity). The column chromatography eluent adopts petroleum ether containing 10% ethyl acetate by volume, and the conversion rate of dimethylphenylsilane into dimethylphenylsilanol is as follows: figure 2 As shown, the gas chromatogram of the product is image 3 As shown in the figure, the retention times of dimethylphenylsilane, dimethylphenylsila...
Embodiment 2
[0052] Embodiment 2, the preparation of compound triphenylsilanol
[0053] In a 10 mL reaction flask, 1 mL of methanol was added, followed by 100 mM triphenylsilane and 1 mg of Au-ZnO, and magnetically stirred at room temperature for 1 h under the induction of visible light. After the reaction, the catalyst is recovered by centrifugation, the organic phase is distilled off under reduced pressure at 30-40°C to remove the organic solvent, and the target compound triphenylsilanol is obtained by column chromatography with petroleum ether containing 5% ethyl acetate by volume (99% conversion, 99% selectivity). The gas chromatogram of the product is as Figure 5 As shown, the retention times of triphenylsilane and triphenylsilanol are 7.71min and 8.42min, respectively. The structural formula of the product is as follows:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com