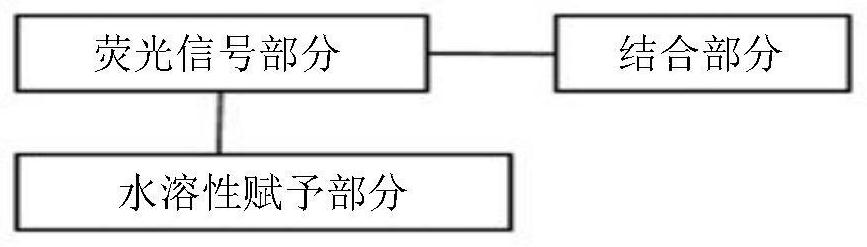
Water-soluble compound for detecting beta-amyloid protein
An amyloid protein and compound technology, which is applied in the directions of preparations, organic chemistry, measuring devices, etc. for in vivo tests, can solve the problems of difficult preparation of water-soluble injections for detecting and diagnosing beta-amyloid, and achieves excellent water solubility. Properties, Effects of Excellent Selective Binding Ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
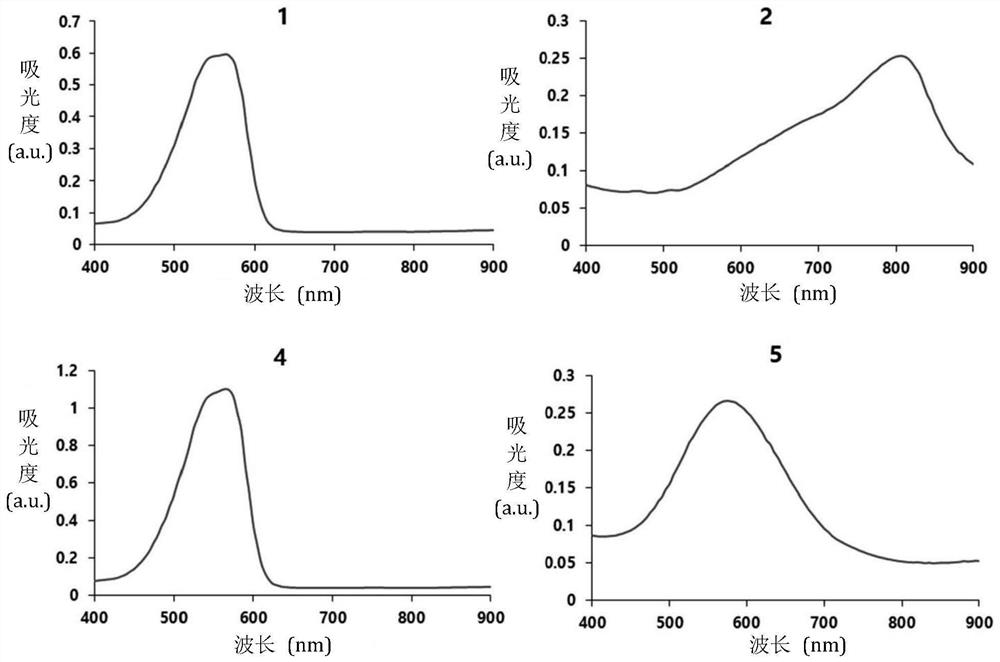
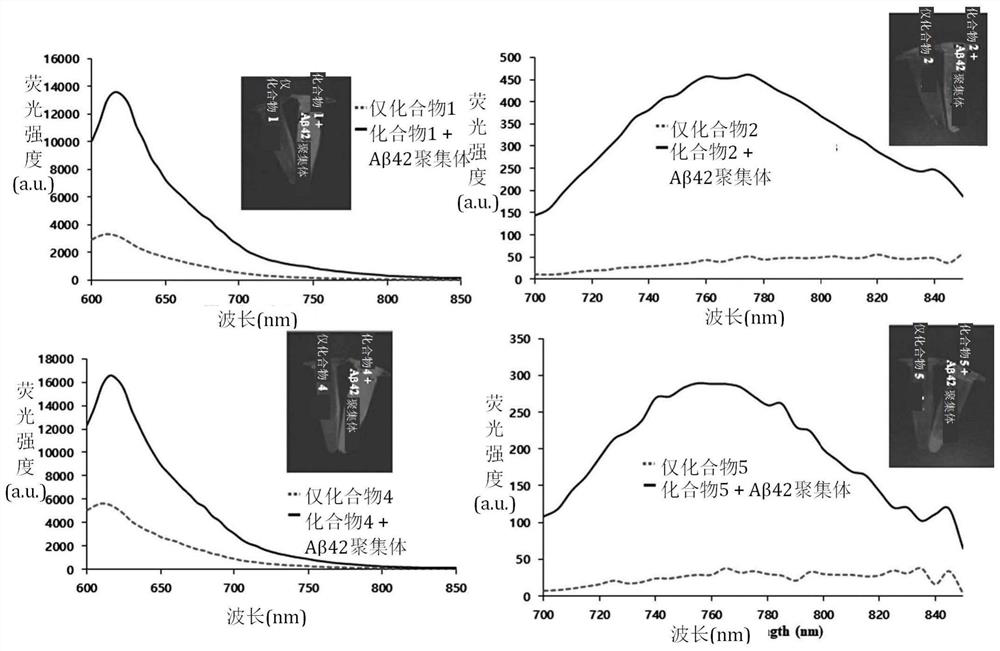
[0086] Example 1 Synthesis of Compound 1
[0087] Compound 1: (E)-3-(2,4-(Dimethylamino)styryl)-1,1-dimethyl-1H-benzo[e]indol-3-cation-3-yl ) butane-1-sulfonate ion
[0088]
[0089] To synthesize compound 1, compound 7 (43 mg, 0.289 mmol) and compound 8 (100 mg, 0.289 mmol) were dissolved in butanol, and the resultant was refluxed at 130°C for 24 hours. The reaction was concentrated under reduced pressure and then chromatographed (CH 2 Cl 2 :MeOH=5:1) was isolated, and compound 1 was synthesized.
[0090] 1 H NMR (500MHz, MeOH-d 4, δ, ppm): 1.80-1.83 (2H, m), 1.93-1.96 (8H, m), 2.51-2.52 (2H, m), 3.13 (6H, s), 4.60-4.63 (2H, m), 6.85 (2H,d,J=9.0Hz),7.37(1H,d,J=15.5Hz),7.61(1H,t,J=7.0Hz),7.72(1H,t,J=7.0Hz),7.99(1H ,d,J=8.0Hz),8.12(2H,d,J=8.0Hz),8.17(1H,d,J=9.0Hz),8.32(1H,d,J=8.5Hz),8.38(1H,d , J=15.5Hz).
Embodiment 2
[0091] Example 2 Synthesis of Compound 2 and Compound 3
[0092] Compound 2: (E)-4-(2-(2-(5-(4-(dimethylamino)phenyl)thiophen-2-yl)vinyl)-1,1-dimethyl-1H-benzene [e]indol-3-cation-3-yl)butane-1-sulfonate ion
[0093] Compound 3: (E)-4-(2-(2-(5'-(4-(dimethylamino)phenyl)-[2,2'-bithiophene]-5-yl)vinyl)-1 ,1-Dimethyl-1H-benzo[e]indol-3-cation-3-yl)butane-1-sulfonate ion
[0094]
[0095] Compound 11 (167 mg, 0.7 mmol) and Pd (PPh 3 ) 4 (80 mg, 0.07 mmol) dissolved in toluene (10 ml). Compound 9 or 10 (0.7 mmol) was dissolved in EtOH (8 ml), and the resultant was added to the mixture. In addition, adding 2M K 2 CO 3 (2 mL), the resultant was refluxed at 120°C for 5 hours, and then concentrated under reduced pressure. The resulting mixture was separated by chromatography (hexane:ethyl acetate=4:1) to synthesize compound 12 or 13.
[0096] Compound 12 or 13 (0.289 mmol) and compound 8 (100 mg, 0.289 mmol) were dissolved in butanol, and the resultant was refluxed at 130° ...
Embodiment 3
[0099] Example 3 Synthesis of Compound 4
[0100] Compound 4: (E)-4-(1,1-Dimethyl-2-(4-(piperazin-1-yl)styryl)-1H-benzo[e]indole-3-cation -3-yl)butane-1-sulfonate ion
[0101]
[0102] Compound 14 (84 mg, 0.289 mmol) and Compound 8 (100 mg, 0.289 mmol) were dissolved in butanol, and the resultant was refluxed at 130° C. for 24 hours. The reaction was concentrated under reduced pressure and then chromatographed (CH 2 Cl 2 :MeOH=5:1) was isolated, and compound 15 was synthesized. Dissolve compound 15 in CH 2 Cl 4 In the solution, TFA was added, and the mixture was stirred at room temperature for 12 hours to obtain compound 4.
[0103] 1 H NMR (500MHz, MeOH-d 4 ,δ,ppm): 1.82-1.83(2H,m), 1.97-1.99(8H,m), 2.53-2.54(2H,m), 3.22-3.24(2H,m), 3.69-3.71(2H,m) ,4.69-4.72(2H,m),7.12(2H,d,J=9.0Hz),7.53(1H,d,J=15.5Hz),7.65(1H,t,J=7.0Hz),7.75(1H, t,J=7.0Hz),8.05(1H,d,J=8.0Hz),8.15-8.22(4H,m),8.35(1H,d,J=8.5Hz),8.43(1H,d,J=15.5 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com