Application of fenretinamide in preparation of medicine for treating or preventing viral hepatitis
A viral hepatitis, the use of technology, applied in the field of antiviral drugs, to achieve good druggability, excellent clinical safety, the effect of removing hepatitis B virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
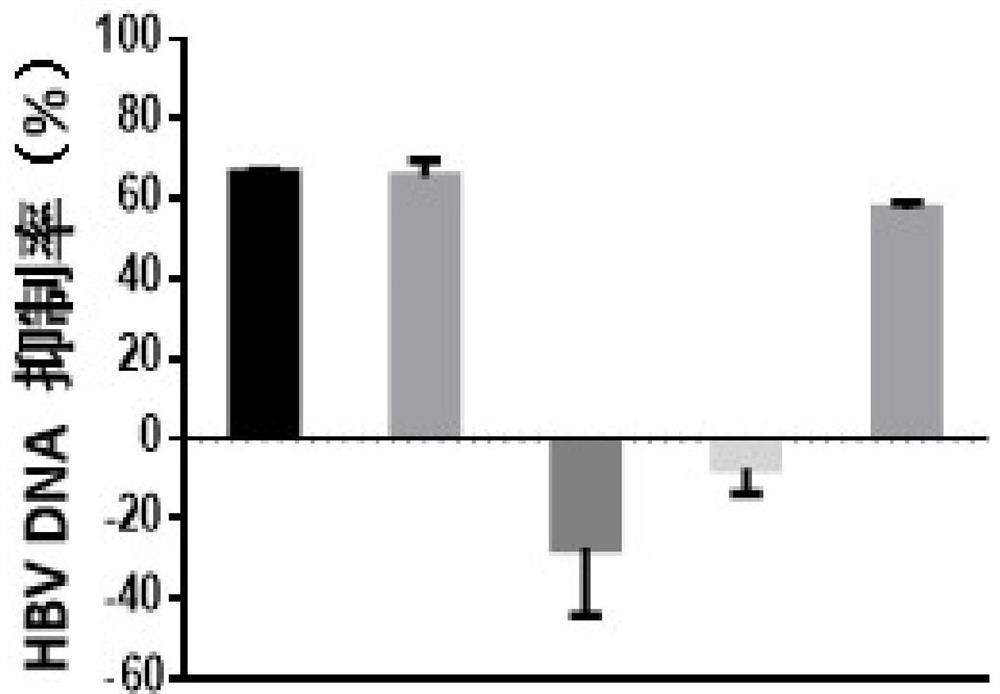
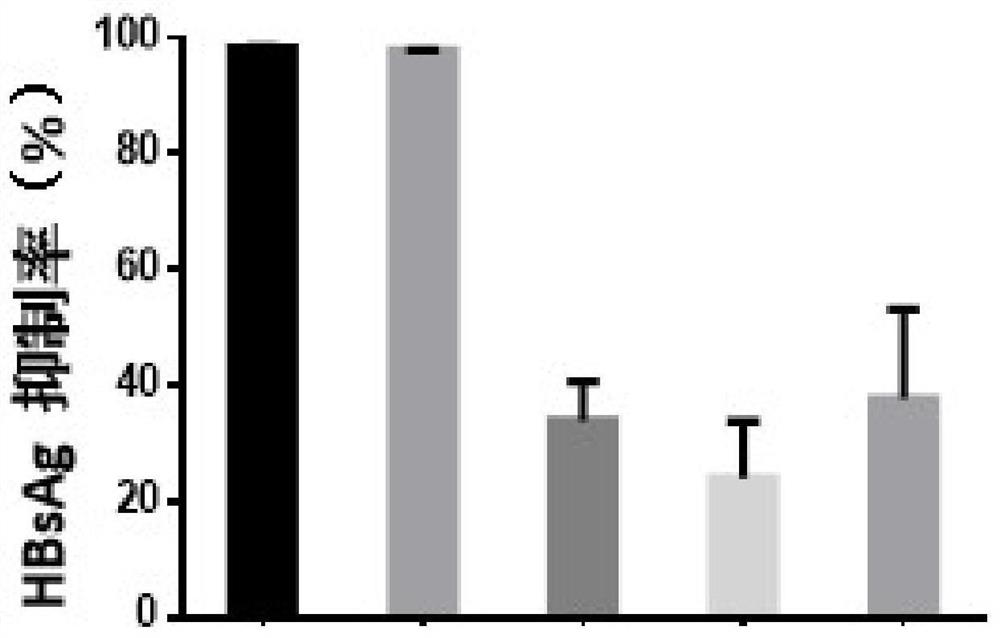
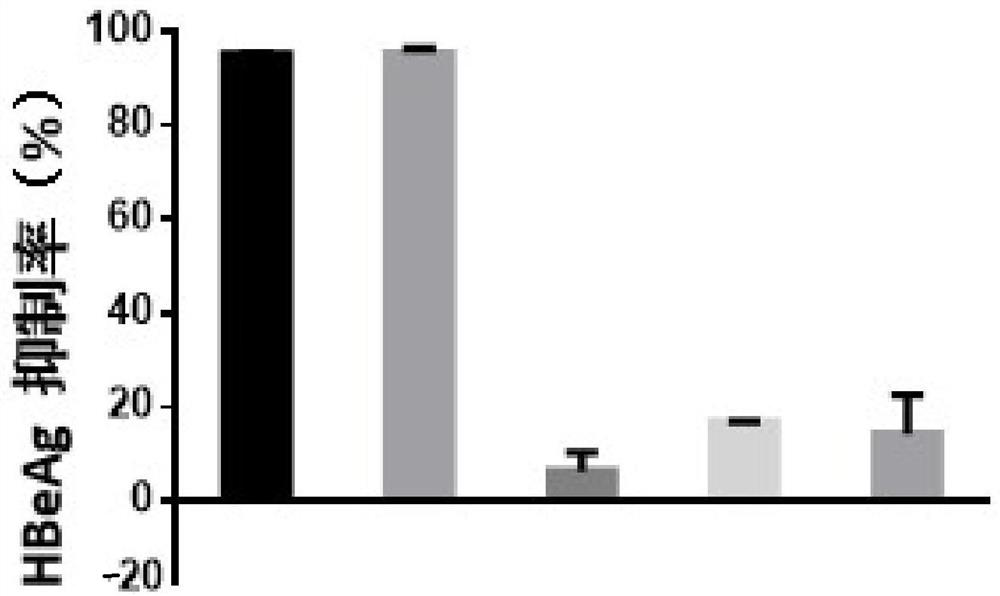
[0068] Example 1 - Evaluation of the in vitro anti-HBV activity of the test compound fenretinide using HepG2-NTCP cells
[0069] The compound preparation method is as follows:
[0070] Taking the preparation of 10mM concentration as an example, the volume of solvent DMSO (μl) = sample mass (mg) × purity ÷ molecular weight ÷ 10 × 10 6
[0071] Control compounds included ETV (lot number: P1214012; 99.0% purity), purchased from Shanghai Titan Technology Co., Ltd.; and RG7834 (RG7834, also known as RO 7020322, a highly selective and orally bioavailable HBV inhibitor with potent Inhibits HBV antigens (HBsAg and HBeAg) and HBV DNA. Lot number: ET25747-14-P1; 99.5% purity), obtained from Shanghai WuXi AppTec New Drug Development Co., Ltd. The stock concentrations of the above control compounds were all 20 mM and stored at -20°C.
[0072] Table 1: Main reagents and cellular viruses
[0073]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com