Application of disodium dimercaptosuccinate in preparation of medicine for treating or preventing viral hepatitis
A dimercaptobutane disodium, viral hepatitis technology, applied in antiviral agents, drug combinations, pharmaceutical formulations, etc., to achieve excellent pharmacokinetic properties, removal of hepatitis B virus, and good druggability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
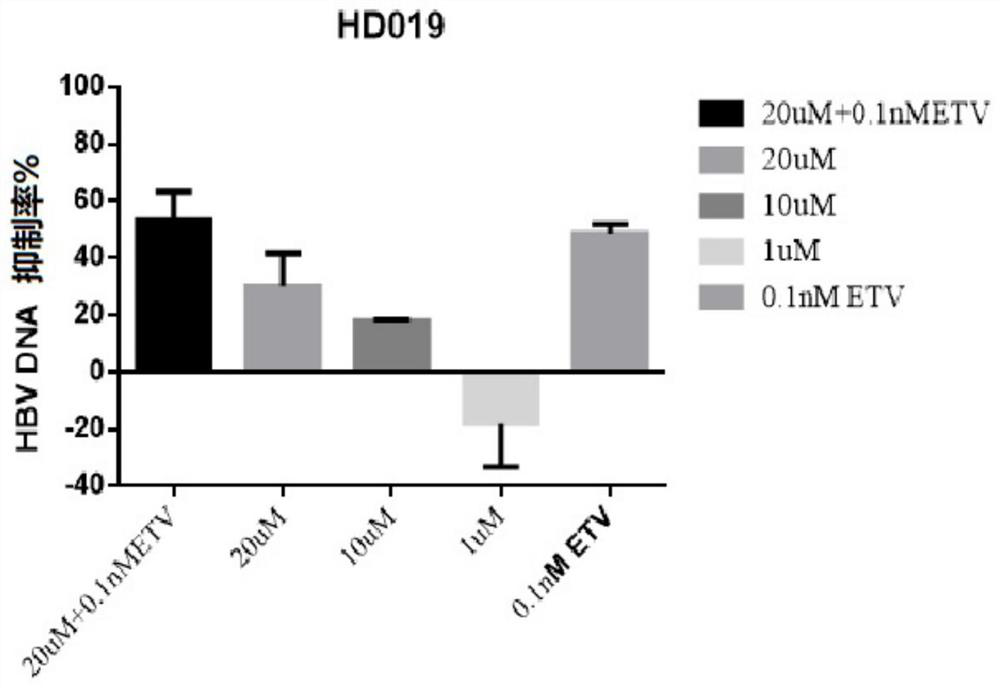
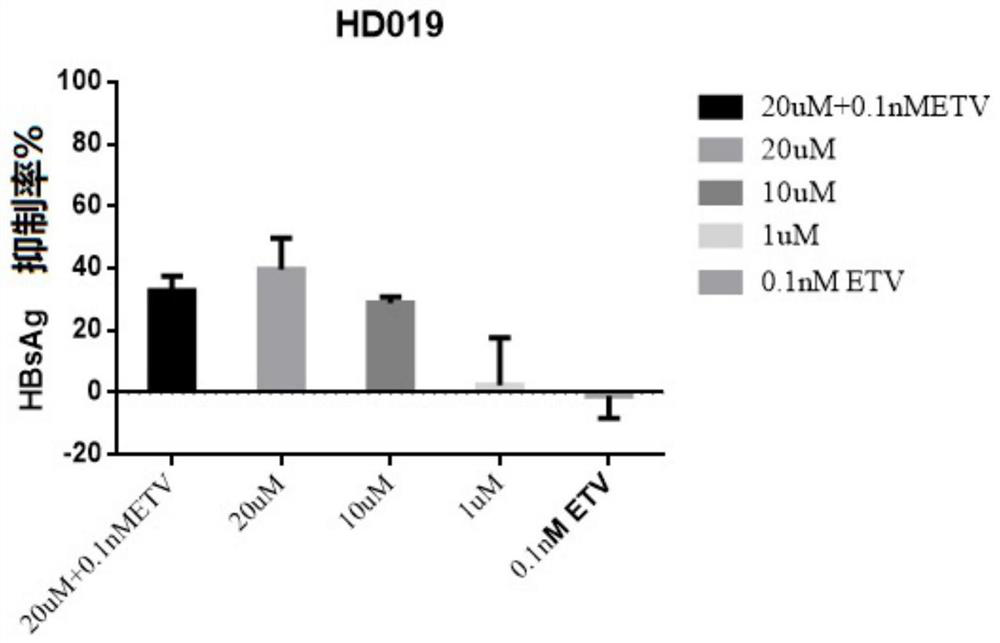
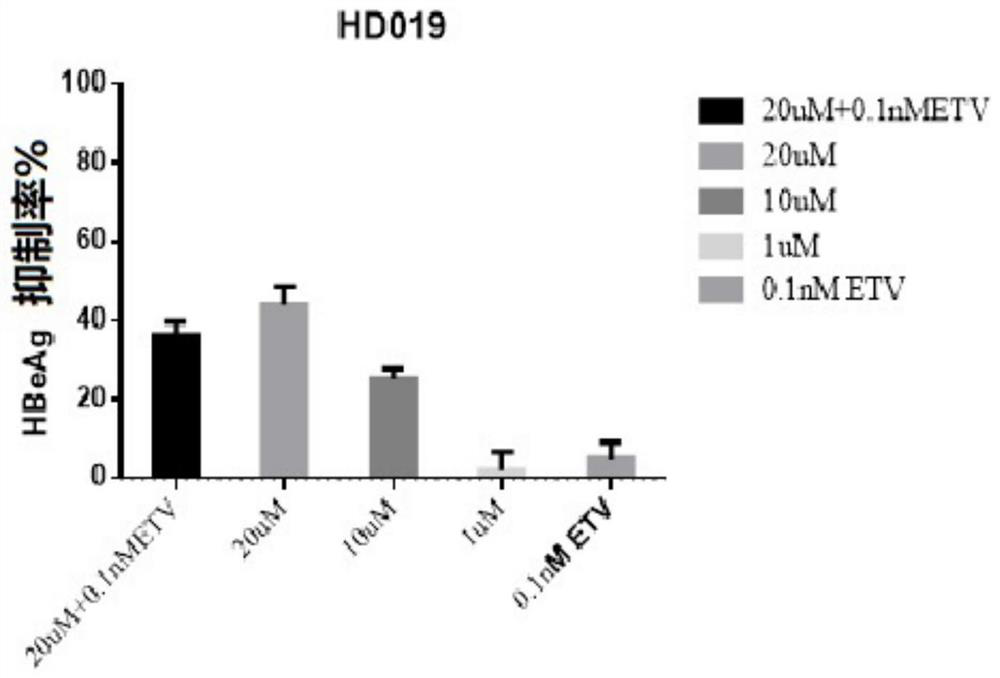
[0067] Embodiment 1-apply HepG2-NTCP cell to evaluate the in vitro anti-HBV activity of test compound dimercapto disodium
[0068] Compound preparation method is as follows:
[0069] Take the preparation of 10mM concentration as an example, the volume of solvent DMSO (μl) = sample mass (mg) × purity ÷ molecular weight ÷ 10 × 10 6
[0070] Control compounds included ETV (lot number: P1214012; 99.0% purity), purchased from Shanghai Titan Technology Co., Ltd.; and RG7834 (RG7834, also known as RO 7020322, is a highly selective and orally bioavailable HBV inhibitor, potent Inhibition of HBV antigens (HBsAg and HBeAg) and HBV DNA. Batch number: ET25747-14-P1; 99.5% purity), obtained from Shanghai WuXi Kangde New Drug Development Co., Ltd. The stock solutions of the above reference compounds were all at a concentration of 20 mM and stored at -20°C.
[0071] Table 1: Major Reagents and Cell Viruses
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com