Pharmaceutical composition for treating viral hepatitis
A composition and drug technology, applied in the field of compounds and pharmaceutical compositions for the treatment or prevention of viral hepatitis, can solve the problems of no direct effect on HBeAg and HBsAg clearance, huge medical costs due to drug resistance, side effects of drugs, and reduction of HBV DNA, etc., to achieve Excellent clinical safety and pharmacokinetic properties, improved clearance of hepatitis B virus, good synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
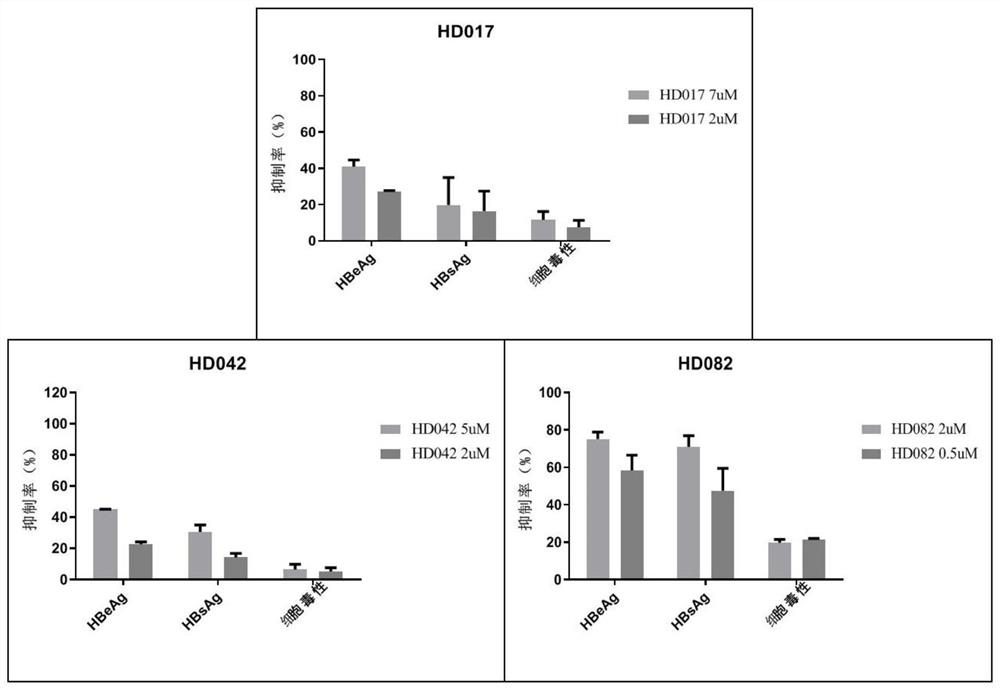
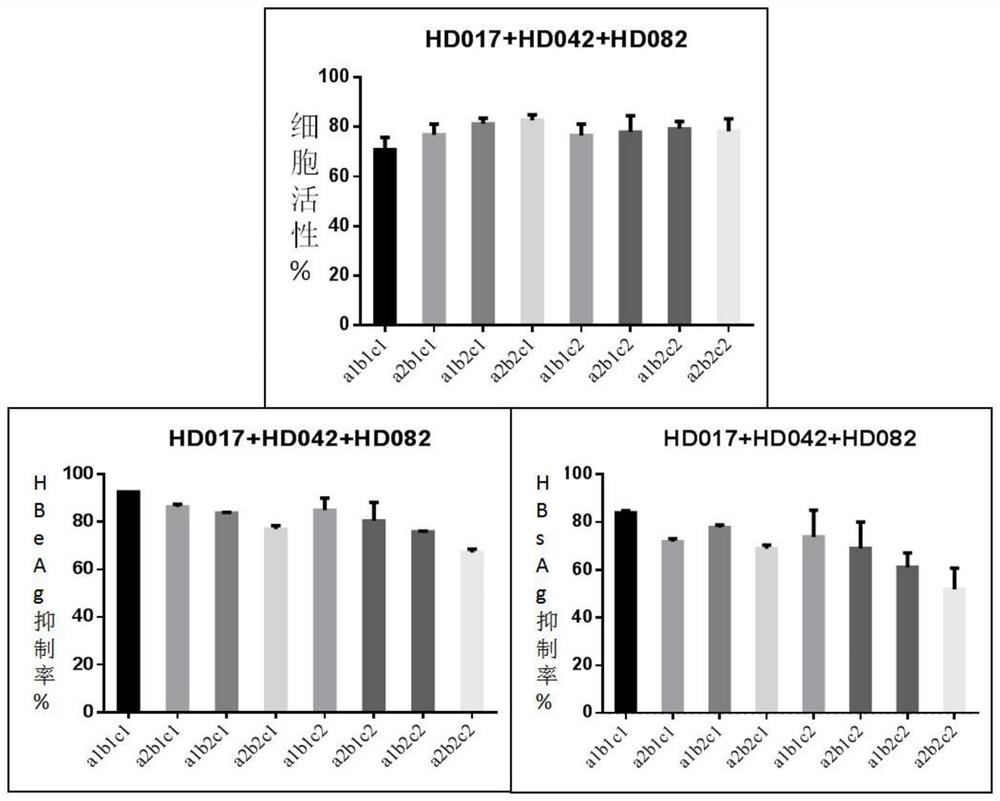
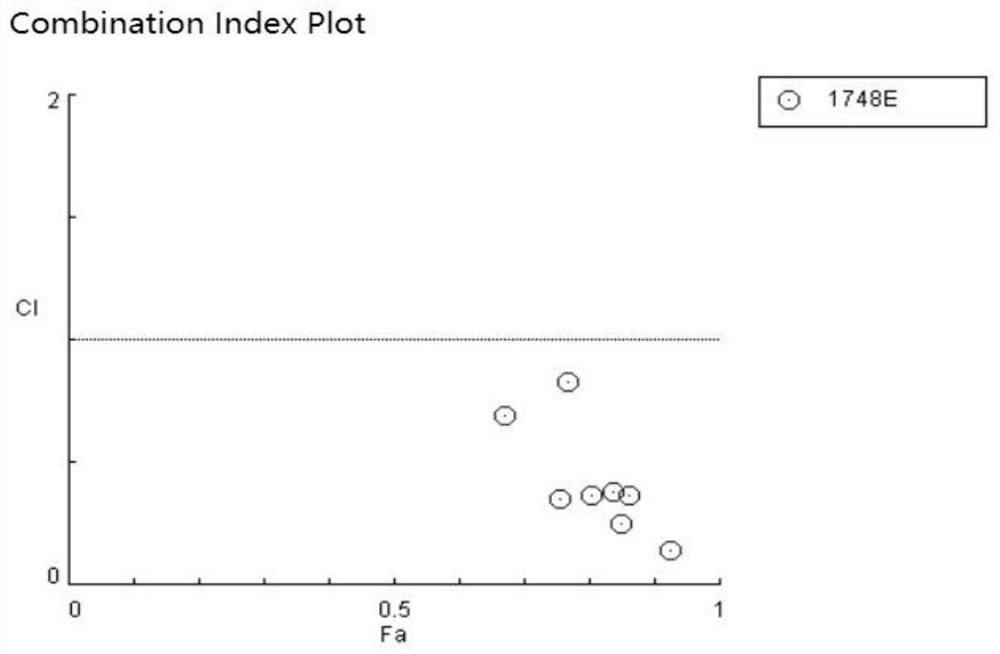
[0056] Evaluation of the synergistic effect of lansoprazole, celecoxib and rapamycin against HBV in vitro using HepG2-NTCP cells
[0057] 1. Compound
[0058] Celecoxib (code HD042), lansoprazole (code HD017) and rapamycin (HD082) were purchased from Shanghai Taosu Biotechnology Co., Ltd.
[0059] Compound preparation method is as follows:
[0060] Take the preparation of 20mM concentration as an example, the volume of solvent DMSO (μl) = sample mass (mg) × purity ÷ molecular weight ÷ 20 × 10 6
[0061] The control compound was entecavir (ETV, batch number: P1214012; 99.0% purity), which was purchased from Shanghai Titan Technology Co., Ltd. The concentrations of the mother solutions of the above compounds were all 20 mM and stored at -20°C.
[0062] 2. Cells and media
[0063] HepG2-NTCP cells: provided by Shanghai WuXi PharmaTech New Drug Development Co., Ltd.
[0064] Freezing PHH culture medium: mainly DMEM medium (Gibco product number 11960051) containing 10% fetal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com