ADA instant detection test strip and kit for adalimumab drug
A technology for detecting adalimumab and test strips, which is applied in the direction of resisting vector-borne diseases, measuring devices, instruments, etc., can solve problems such as unsuitable popularization, time-consuming and labor-consuming, and long time required to obtain results, and achieve results The effect of rapid interpretation, no need for equipment, and reduced operation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
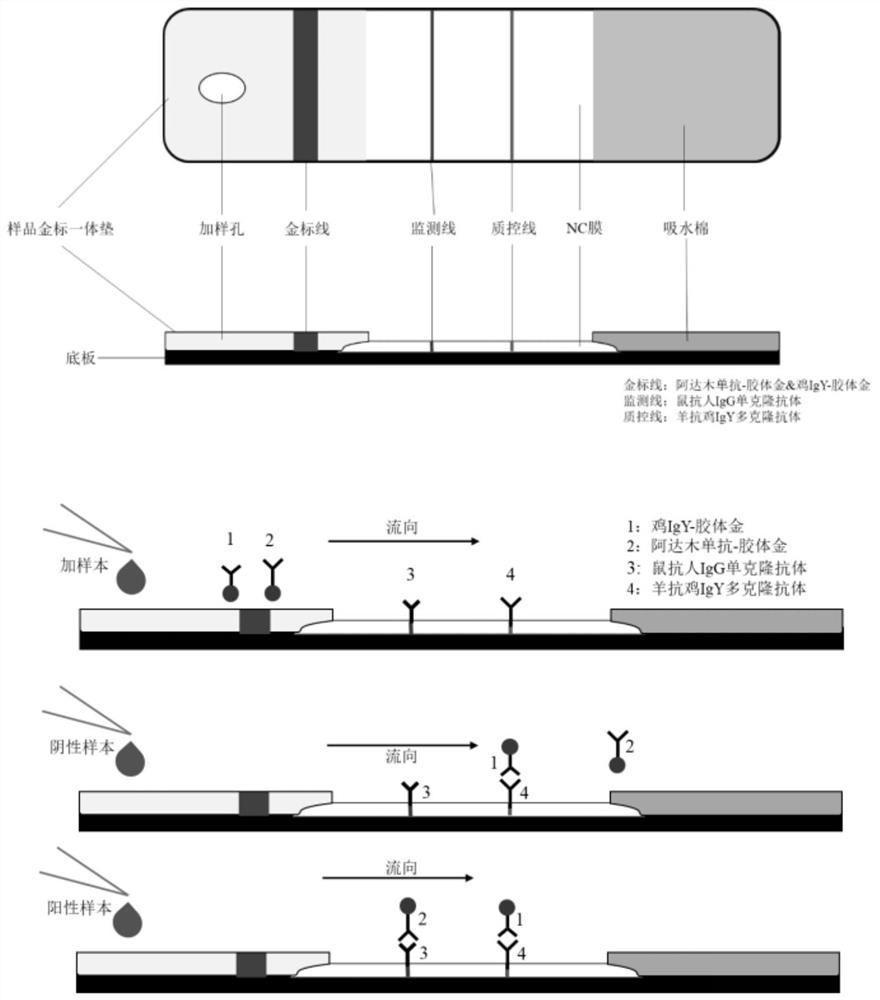
[0046] Preparation of ADA point-of-care test strips and kits for adalimumab:
[0047] 1. Gold spraying treatment: The adalimumab drug-45nm colloidal gold conjugate and chicken IgY monoclonal antibody colloidal gold conjugate were sprayed evenly on the sample gold label at a concentration of 4 μL / cm and 1 mg / mL respectively. On the one-piece pad, diluent I (0.1M Tris, 1% BSA, 2% sucrose, pH 8.0) was placed at 37°C for overnight treatment.
[0048] The preparation methods of adalimumab colloidal gold conjugate and chicken IgY monoclonal antibody colloidal gold conjugate are as follows:
[0049] Add 20ml of the prepared colloidal gold particles to the beaker, add 100μl of 0.1M potassium carbonate, rotate at 200 rpm on a magnetic stirrer, and react for 5min;
[0050] Add 100 μl of 1 mg / ml antibody to be labeled into the beaker, and react at 200 rpm on a magnetic stirrer for 30 min;
[0051] Add 200 μl of 10% BSA to the beaker, and react for 15 min on a magnetic stirrer at 200 rp...
Embodiment 2
[0058] Use the adalimumab antidrug antibody detection kit of Example 1 to confirm the critical point. The method of use is as follows: place the test strip horizontally, add 10ul of the sample to the sample addition hole, and then dropwise add 2 to 3 drops of the sample diluent , and observe the results after standing for 15 minutes.
[0059] The results are determined as follows:
[0060] Positive: A red line appears on each of the quality control line (hereinafter referred to as C line) and the detection line (hereinafter referred to as T line), indicating the presence of adalimumab ADA in the sample, and the test result is positive.
[0061] Negative: Only the quality control line (C line) has a red line, and the detection line (T line) has no red line, indicating that there is no adalimumab ADA in the sample or the ADA is below the detection level, and the test result is negative.
[0062] Invalid: There is no red line on the control line (C line), which means it is inval...
Embodiment 3
[0072] Positive sample confirmation:
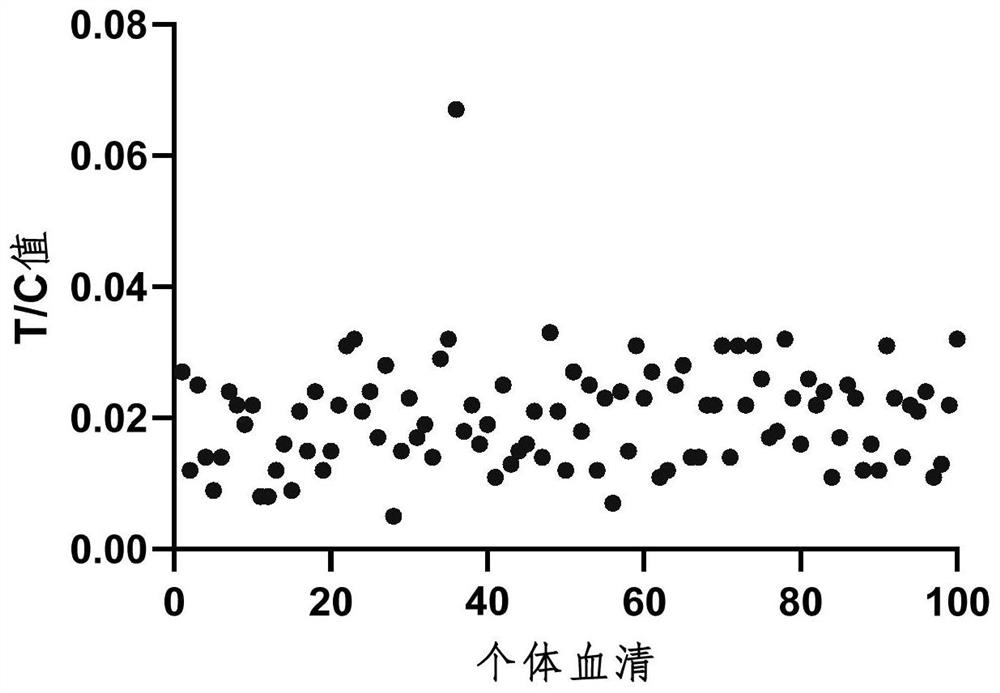
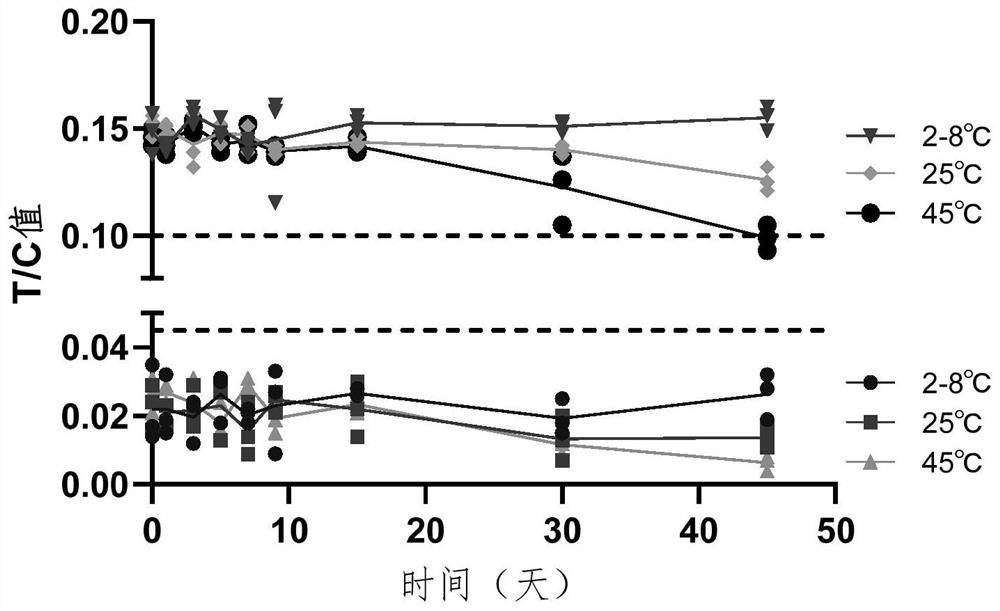
[0073] Since this method is mainly based on operator observation and judgment, in order to further explore the disputed range between T / C value and the operator's direct observation and judgment results, and to ensure that the direct interpretation results are all positive samples, the experiments were conducted by preparing positive samples with different concentrations. The experimental operation was performed according to the operation flow of Example 3. The experimental results are shown in Table 2.
[0074] Table 2-1 Test results of positive samples
[0075]
[0076] Table 2-2 Test results of positive samples
[0077]
[0078] From the above test results, it was found that when the ADA concentration was 50ng / mL, there was no objection to the interpretation results, and they were all judged as positive results (Table 2-1 and Table 2-2). In order to further confirm the critical area of the operator's direct interpretation of ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap