Intranasal Dantroline Administration For The Treatment Of Alzheimer Disease
An Alzheimer's, intranasal technology for neurological diseases, medical preparations containing active ingredients, pharmaceutical formulations, etc., which can solve the limitations of new effective drug development, insufficient cell or animal models, lack of SAD mechanism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
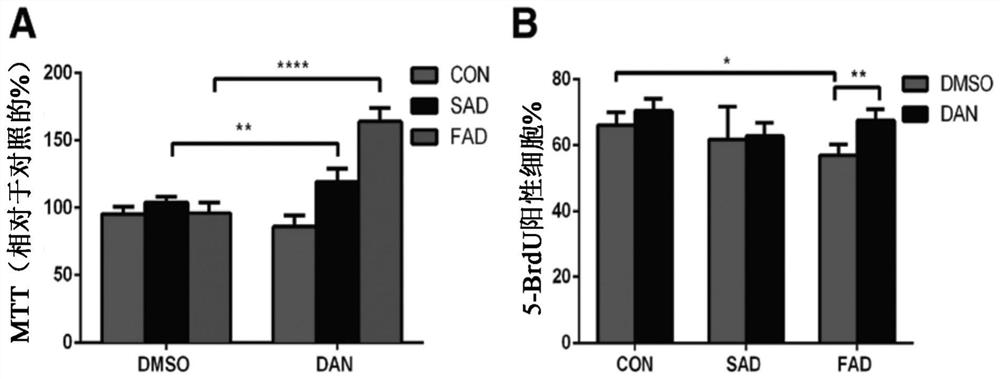
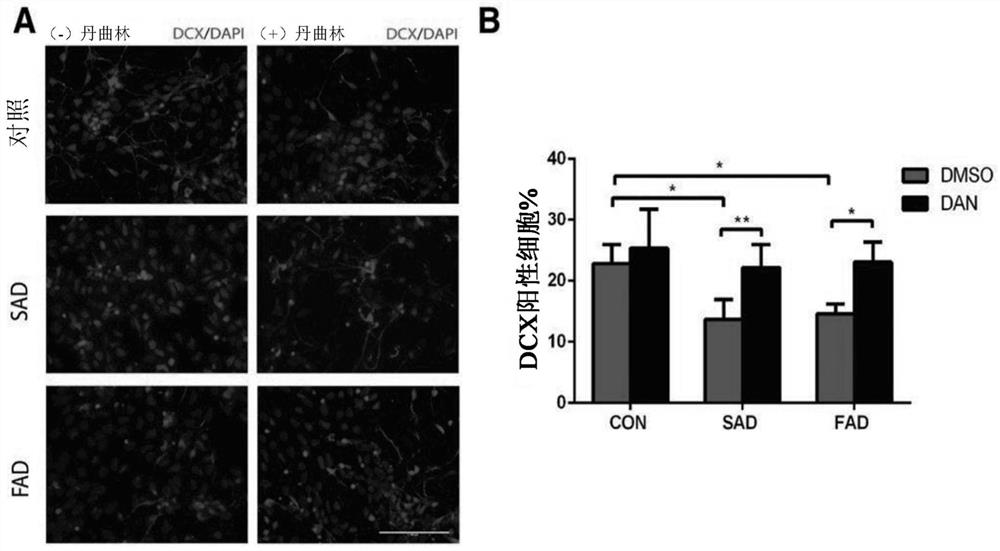
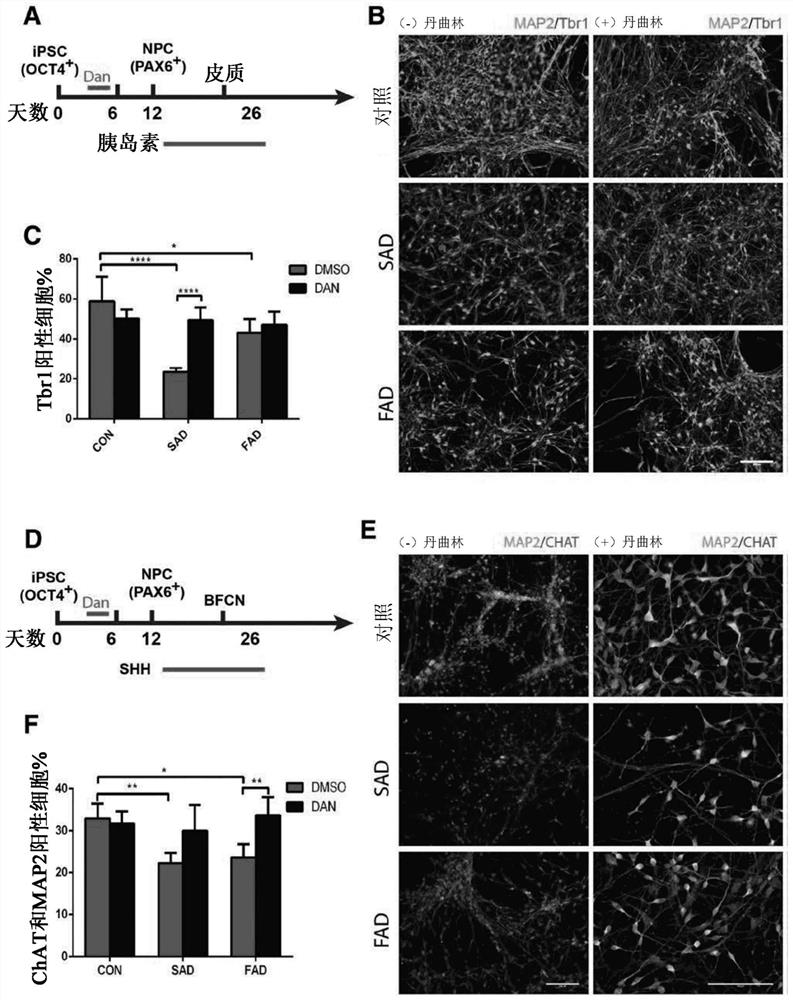
[0067] Dantrolene inhibits impaired neurogenesis and synaptogenesis in induced pluripotent stem cells from Alzheimer's disease patients
[0068] While not wishing to be bound by any particular theory, it is believed that dantrolene inhibits impaired neurogenesis by correcting calcium dysregulation due to ryanodine receptor overactivation and associated impaired lysosomal and autophagic function. synapses occur. In this study, and by using iPSCs and their derived neural progenitor cells (NPCs) and basal forebrain cholinergic neurons (BFCNs) from both SAD patients and FAD patients, the effect of dantrolene on neurogenesis was investigated and the effects and mechanisms of synaptogenesis. Dantrolene significantly ameliorated neurogenesis and synaptogenesis impairment, which was related to correcting RyR hyperactivation, intracellular Ca 2+ Dysregulation is associated with disruption of autophagy.
[0069] Materials and Methods
[0070] cell culture
[0071] Human control (AG...
example 2
[0112] Intranasal administration of dantrolene increases its concentration and duration in the brain
[0113] Intranasal administration of dantrolene is proposed as a novel therapeutic approach to maximize the potential neuroprotective effects of dantrolene in various neurodegenerative diseases, especially AD, while minimizing its toxicity and side effects. As described herein, this study shows that intranasal administration of dantrolene in mice significantly increases the concentration and duration of dantrolene in the brain compared to the usual oral administration.
[0114] Materials and Methods
[0115] animal
[0116]All procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Male and female C57BL / 6 mice, 2-4 months old, weighing 25-35 g, were used in all experiments. Mice were maintained at 21-22°C on a 12-hour light-dark cycle with food and water ad libitum. All efforts ...
example 3
[0145] Intranasal dantrolene as a disease-modifying drug in Alzheimer's disease 5XFAD mice
[0146] This study investigated plasma and brain concentrations in 5XFAD mice and the therapeutic efficacy and associated side effects of intranasal dantrolene, not only as a symptom-relieving drug but also as a disease-modifying drug, and compared with that in different FAD animals. The subcutaneous approach performed in the model was compared as described by Peng J et al., Neuroscience Letters Letters 2012;516:274, which is incorporated by reference in its entirety.
[0147] Materials and Methods
[0148] animal
[0149] All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Two pairs of 5XFAD mice (B6SJL-Tg(APPSwFIL on, PSEN1*M146L*LV286V)6799Vas / Mmjax) and wild-type mice (B6SJLF1 / J) mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed. These 5XFAD transgenic mice overexpress mutant human A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com