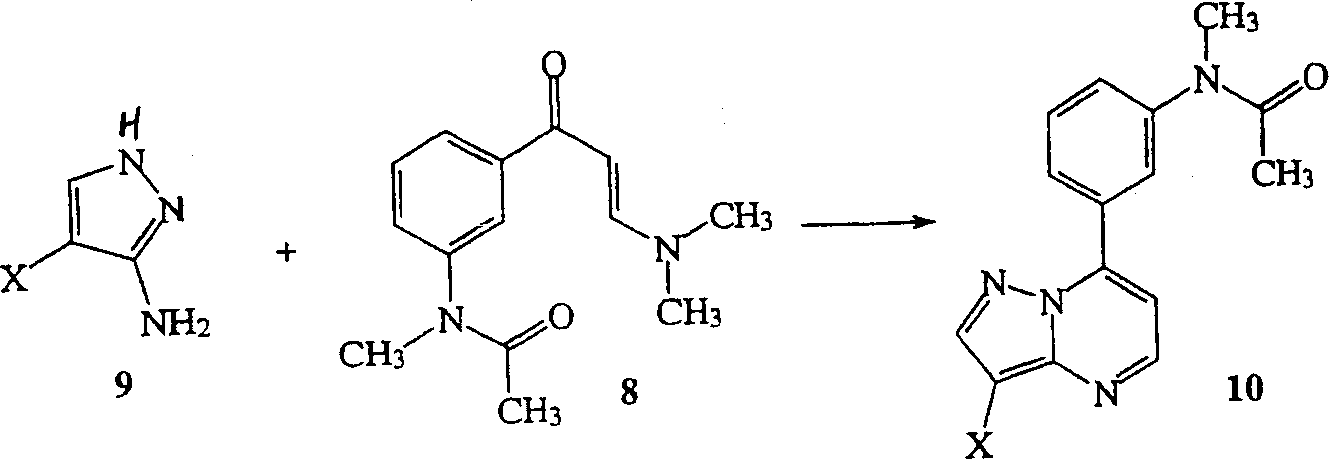
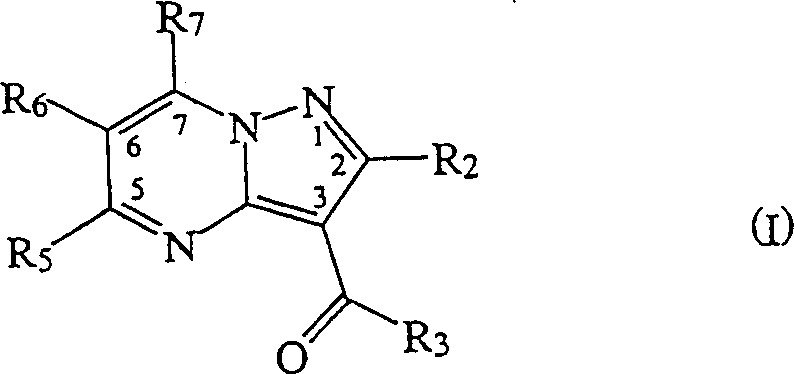
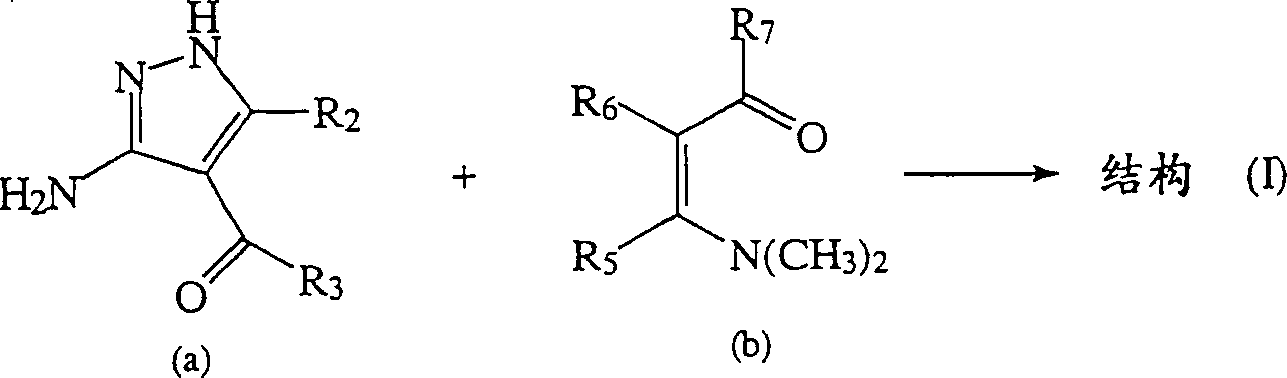
Synthese of substitutd pyrazolopyrimidines
一种唑并嘧啶、吡唑的技术,应用在吡唑并嘧啶的合成领域,能够解决耗时、大规模的合成不是特别经济或有效的等问题,达到简化反应条件、好产率、提高效率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0141] Formation of enaminone 8 of reaction schemes 1, 2 and / or 3 under phase transfer conditions
[0142]
[0143] To enaminone 8' (25 g, 107.6 mmol) in benzotrifluoride (92 mL) and dichloromethane (160 mL) was added tetrabutylammonium sulfate (2 g, 5.9 mmol). Dimethyl sulfate (16 g, 126.7 mmol) was added to the mixture. To this mixture was added 200 g of a 50% aqueous sodium hydroxide solution, and the mixture was vigorously stirred for 6 hours, keeping the reaction temperature below 40°C. Depending on the consumption of starting material, 200 mL of water was carefully added to keep the reaction temperature below 40°C. The aqueous phase was separated off and the organic phase was washed three times with water and dried over magnesium sulfate. The dichloromethane was removed in vacuo to give a yellow solid. The solid was filtered, washed with benzotrifluoride and dried under vacuum to give the alkylated enaminone 8 (19 g, 72% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com