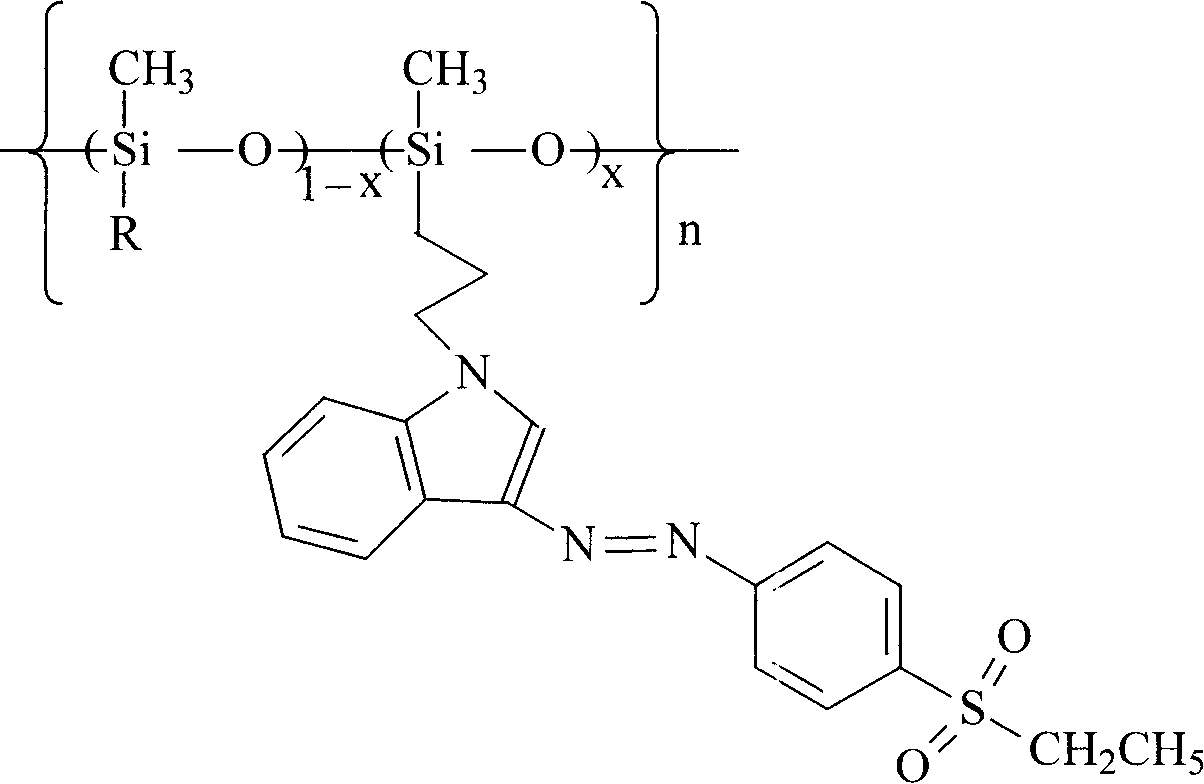
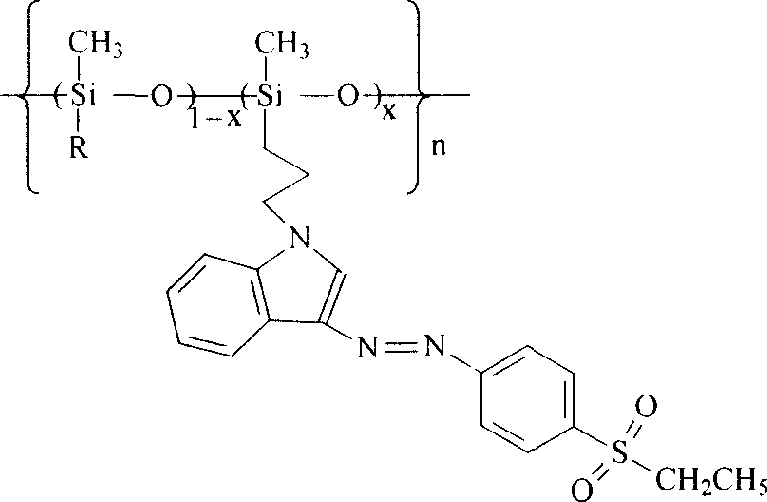
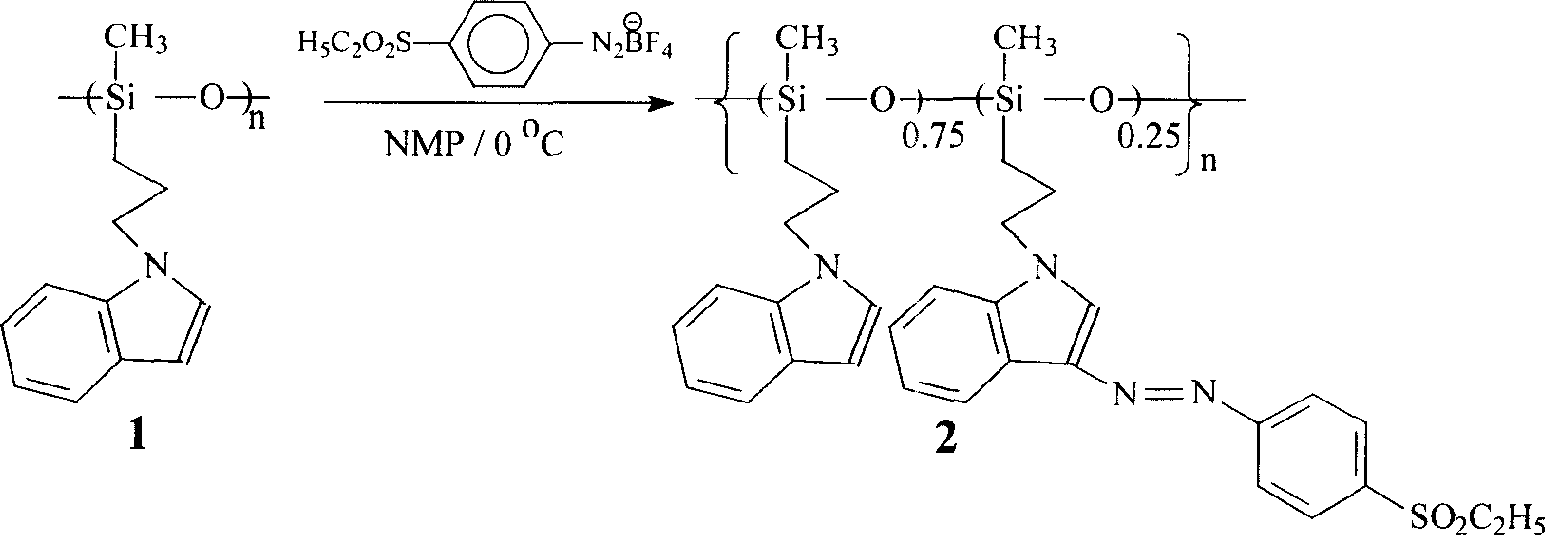
Polysiloxane with sulphonyl indole chromophore side group, its preparation and application
A technology of polysiloxane and sulfone-based azoindole, which is applied in instruments, optical components, optics, etc., can solve the problems of no indole chromophore and no polymer, and achieve convenient purification and mild reaction conditions , the effect of rich content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] R is an indolyl substituted propylene group, when x is 0.25, the synthetic route is as follows:
[0018]
[0019] The synthesis method is:
[0020] Dissolve p-ethylsulfonyl aniline (2.79g, 0.015mol) in 9.90g 40% fluoroboric acid (about 0.045mol), cool to 0-5°C in an ice bath, slowly add sodium nitrite (1.04g, 1.04g, 0.015 mol) dissolved in 4 mL of water, and the reaction temperature is controlled below 5°C. After half an hour of reaction, the filter cake was quickly filtered with suction. The filter cake was washed several times with cold absolute ethanol and anhydrous ether. After draining, 4.05g of light yellow powder was obtained, which was p-ethylsulfonyl aniline diazofluoroborate. The yield 95%, put in the refrigerator for later use.
[0021] In a Schlenk tube equipped with a stirring magnet, add hydrogen-containing silicone oil, N-allyl indole, a little dichlorodicyclopentadiene platinum catalyst, anhydrous toluene, and at 60°C under argon protection The reaction wa...
Embodiment 2
[0024]
[0025] R is an indolyl-substituted propylene group, when x is 0.21, the synthetic route is as follows:
[0026] The synthesis method is as follows: Weigh the above 250 mg polysiloxane polymer 1, put it into a 30ml test tube, add N-methylpyrrolidone (make the solution mass percentage concentration 8-15%), stir at room temperature until the polymer is complete Dissolve in an ice bath. After the temperature of the solution drops to 0℃, add 66 mg of p-ethylsulfonyl aniline diazofluoroborate, react for 8 hours under ice bath conditions, and then add 0.2 g of anhydrous K 2 CO 3 Keep the ice bath conditions, continue to stir for 1 hour, filter, wash the reaction tube with tetrahydrofuran, collect the filtrate, extract the tetrahydrofuran under reduced pressure, add 20ml methanol to obtain a red precipitate, filter, collect the red solid, wash with methanol several times, vacuum After drying, the desired second-order nonlinear optical polysiloxane (poly(indolyl) (sulfone azoind...
Embodiment 3
[0028] R is indolyl substituted propylene and carbazolyl substituted propylene, when x is 0.17, the synthetic route is as follows:
[0029]
[0030] The synthesis method is: in a Schlenk tube equipped with a stirring magnet, add hydrogen-containing silicone oil, N-allyl indole, a little dichlorodicyclopentadiene platinum catalyst, anhydrous toluene, and under argon protection conditions The reaction was stirred at 60°C for 48 hours, cooled to room temperature, then N-allylcarbazole was added, and the reaction was continued to stir at 60°C for 60 hours under argon protection, and a large amount of solvent was evaporated under reduced pressure. Then add appropriate amount of methanol, put it in the refrigerator and freeze for two hours, pour out the supernatant, dissolve the precipitate in a small amount of chloroform, and then re-precipitate with methanol, so several times, a light white solid polysiloxane 4, 40℃ Vacuum drying. Wherein, the molar ratio of the hydrogen-containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com