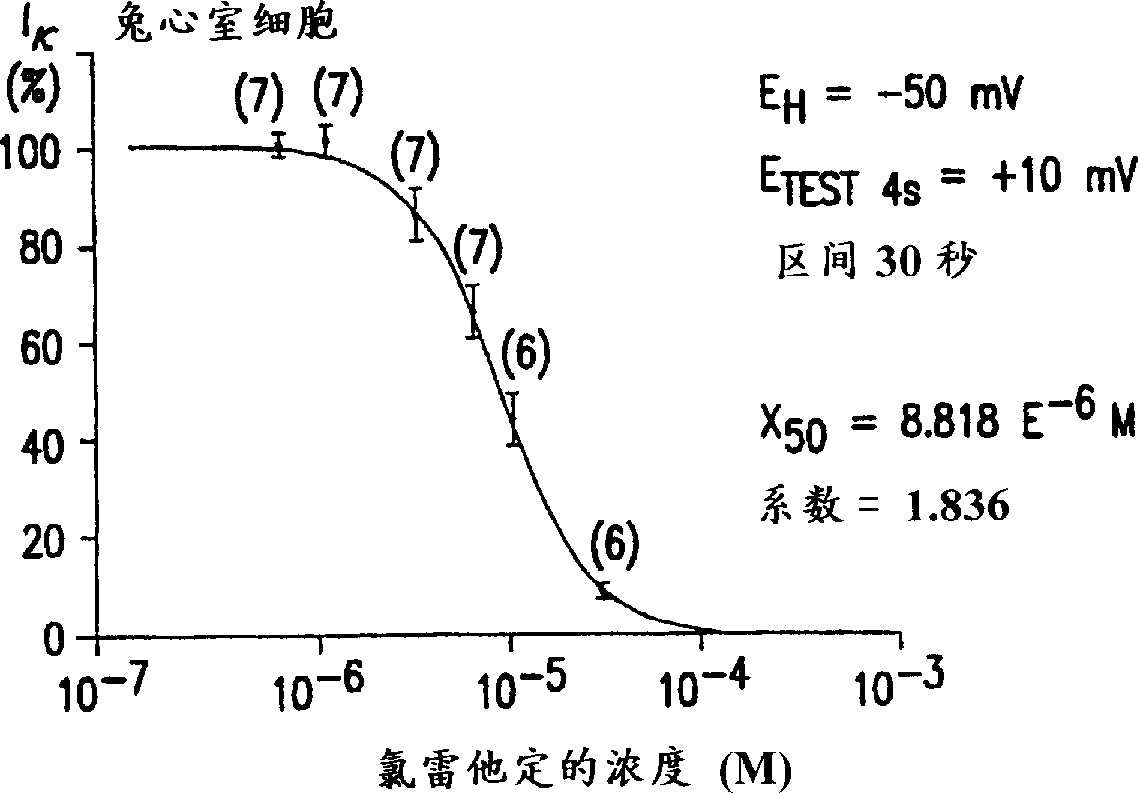
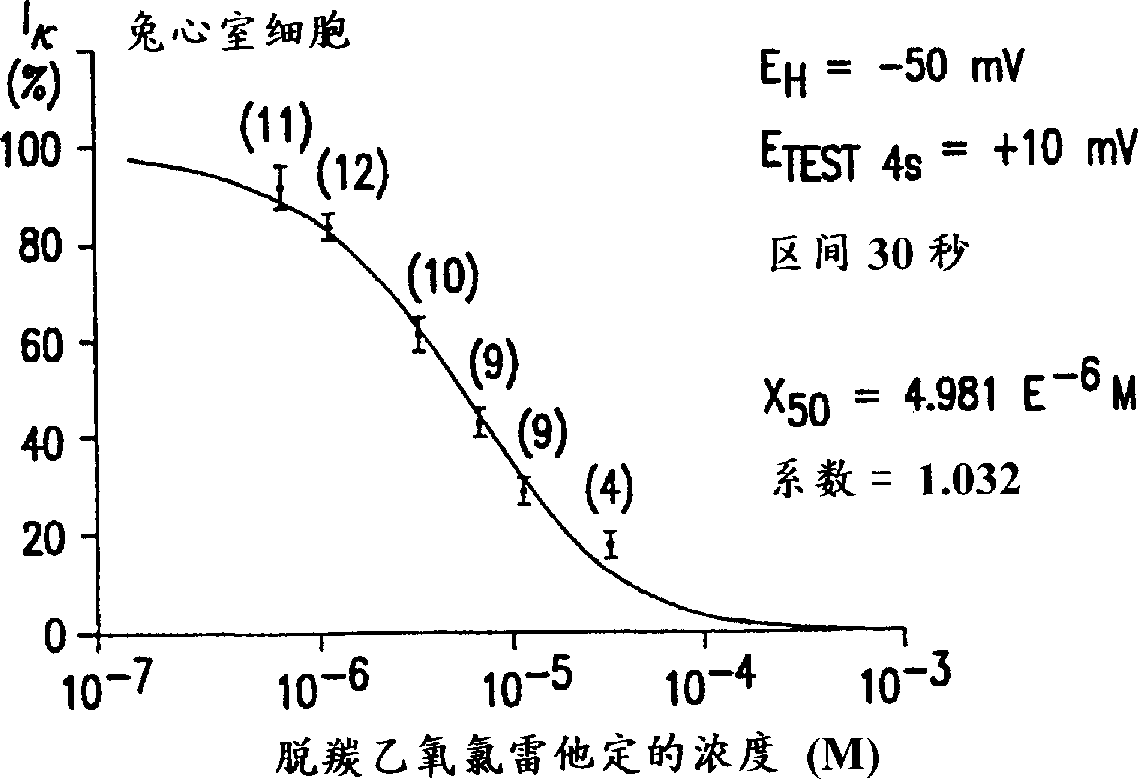
Use of descarboethoxyloratadine for manfacture of medicament for treatment of urinary incontinence, motion sickness and vertigo
A technology for motion sickness and urinary incontinence, applied in the field of dizziness, motion sickness, and urinary incontinence, and can solve problems such as adverse interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Preparation of loratadine and its metabolites
[0103] For example, loratadine can be synthesized by the method disclosed in US Patent 4,282,233. Its metabolites are prepared by reaction procedures routine in the art, as described in US Patent 4,659,716. A common method for preparing DCL is refluxing loratadine in the presence of sodium hydroxide and ethanol as described below. commercialized Kemin _ Extraction of tablets (600 x 10 mg): Loratadine tablets were diluted with water and chloroform. After stirring the mixture was filtered through Celite, rinsing with chloroform until the filtrate was free of loratadine. The separated aqueous layer was extracted twice with chloroform. The combined organic layers were washed with water, brine and dried over sodium sulfate. Evaporation of the solvent gave pure loratadine as a white solid.
[0104] Saponification of Loratadine:
[0105] Loratadine (4.0 g) was added to a solution of sodium hydroxide (5.9 g) in absolute et...
Embodiment 2
[0107] Muscarinic receptor binding studies
[0108] The aim of this study was to identify six compounds that are effective in human m 1 、m 2 and m 3 Affinity of muscarinic receptor subtypes. The method used here is similar to that disclosed by Dörje et al. in The Journal of Pharmacology and Experimental Therapeutics 256:2727-733 (1991).
[0109] method:
[0110] Prepare samples and evaluate human m expressed on mammalian CHO cells over a range of concentrations (0.1-3000 nM, half log dilution values) 1 -m 3 recombinant receptors. These data were generated from binding assays of radiolabeled ligands in which [ 3 H] pirenzepine for m 1 , [ 3 H]AF-DX384 for m 2 , [ 3 H] 4-DAMP (4-diphenylethoxy-N-methylpiperidine) for m 3 .
[0111] After incubation the test substances were rapidly filtered under vacuum through GF / B glass filters (packard) and washed with ice-cold buffer using a Packard cell harvester. Bound radioactivity was measured in a liquid scintillation count...
Embodiment 3
[0122] Tumor Promoting Activity:
[0123] Inhibition of lymphocyte mitogenicity was used to screen the efficacy of loratadine and DCL as tumor promoting agents.
[0124] Mitosis Research:
[0125] Fresh splenocytes (5×10 5 ) were suspended in RPMI 1640 matrix (Grand Island Biological Co. Grand Island NY) containing 2% fetal bovine calf serum, and then inoculated in the matrix to which concanavalin (Con) A (2 μg / ml; Sigma Chemical Co. ., St. Louis, MO) replica microplates (Nuuc). Cells were incubated in the presence or absence of increasing concentrations of test drug in saline or other media (37°C, 95% air, 5% CO 2 ). 43 hours after the addition of concanavalin A, 0.25 nmol[ 3 H]-thymidine (6.7 Ci / nmol; ICN Radiopharmaceuticals, Montreal, PQ) was added to each well. After an additional 5 h incubation, cells were eluted from the wells onto filter paper using an automated cell sorter. Filtrates were placed in vials containing 5 ml of scintillation fluid (Readysafe, Beckma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com