Surface antigens and proteins useful in compositions for diagnosis and prevention of lyme disease
A technology of composition and fusion protein, applied in the direction of peptide/protein composition, antibacterial immunoglobulin, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Example 1 Borrelia strains and antibodies
[0145] A. Bacterial strains
[0146] The B. burgdorferi (narrow sense) JD1 strain was obtained from the Center for Disease Control and Prevention [J. Piesman et al., J. Clin. Microbiol., 25:557-558 (1987) and T.G. Schwan et al. , J. Clin. Microbiol., 27:1734-1738 (1989)] and low passage (<7) frozen stocks were preserved. B31 is also a strain of B. burgdorferi (narrow sense) purchased from CDC and deposited as described above. IP90 is a strain of B. garinii, which was also provided by CDC.
[0147] B. burgdorferi organisms were grown at 34°C in BSK-H medium (Sigma Chemical Co., St. Louis, Mo).
[0148] B. Monkey Antibody
[0149] Serum antibodies were incubated with live spirochetes from macaques that had been infected with B. burgdorferi strain JD1 through pupal bites of Ixodes scapularis. Unbound antibody was removed by washing from the spirochetes, and bound antibody was removed with a low pH buffer. Monkey antibodies ...
Embodiment 2
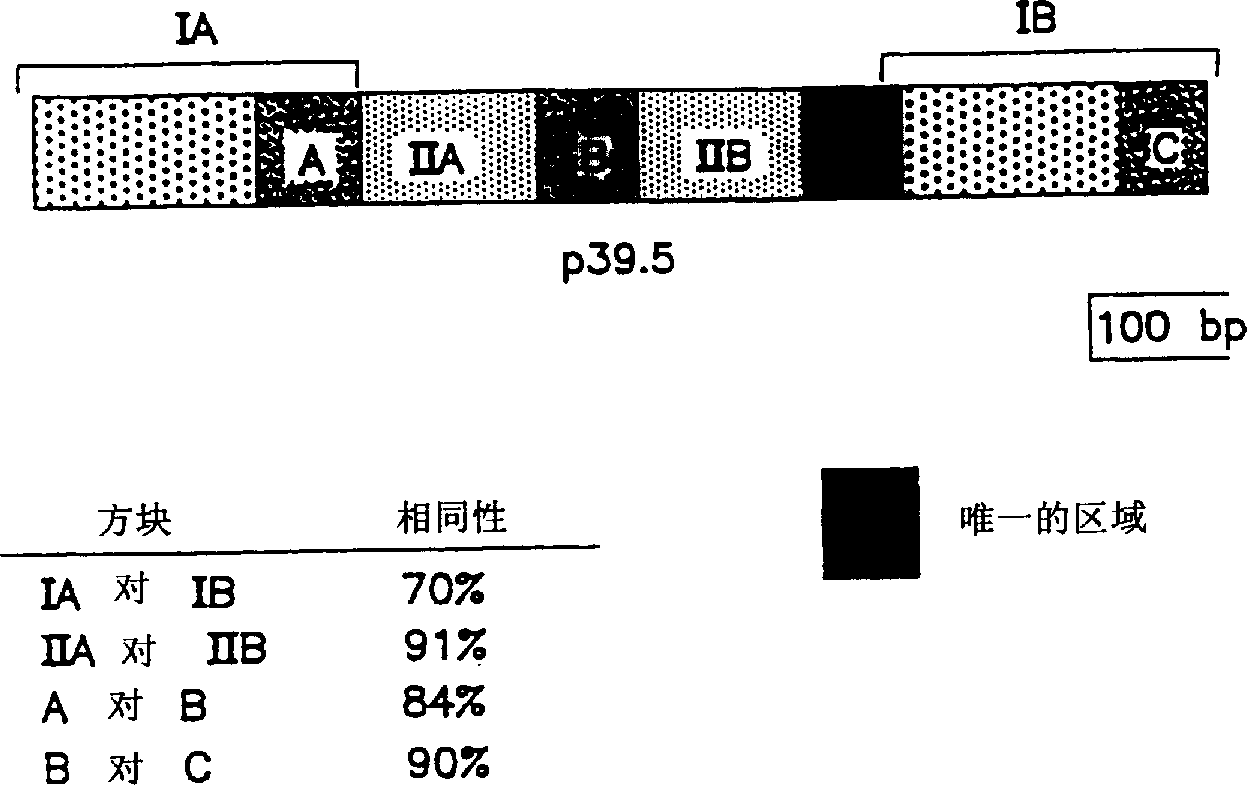
[0153] Example 2 Preliminary Identification of P39.5 of IP90
[0154] Antibodies from part B of Example 1 were reacted with Western blots of whole extracts of JD1, B31 and IP90 spirochetes. Western blotting was performed by electrophoresis of antigen preparations on 15% acrylamide mini gels (10 x 10 x 0.1 cm) and 5% acrylamide stacking gels. Dispense 20 µl per lane containing 7 x 10 8 Bacterial lysate or 25 micrograms of protein (measured at 280nm OD) (there were 16 single lanes across the preparative lane; therefore 400 micrograms of protein were loaded on each preparative gel). Electrophoresis was performed using a buffer in U. Laemmli, Nature, 227:680-685 (1970) at a constant pressure of 23 mA using a mini gel apparatus (Integrated Separation Systems, Hyde Park, MA). For immunoblotting, 22 volts were used in a Mighty Small transfer unit (Hoeffer Scientific Instruments, San Francisco, CA) as described by H. Towbin et al., Proc. Natl. Acad. Sci. USA 76:4350-4354 (1979). Pr...
Embodiment 3
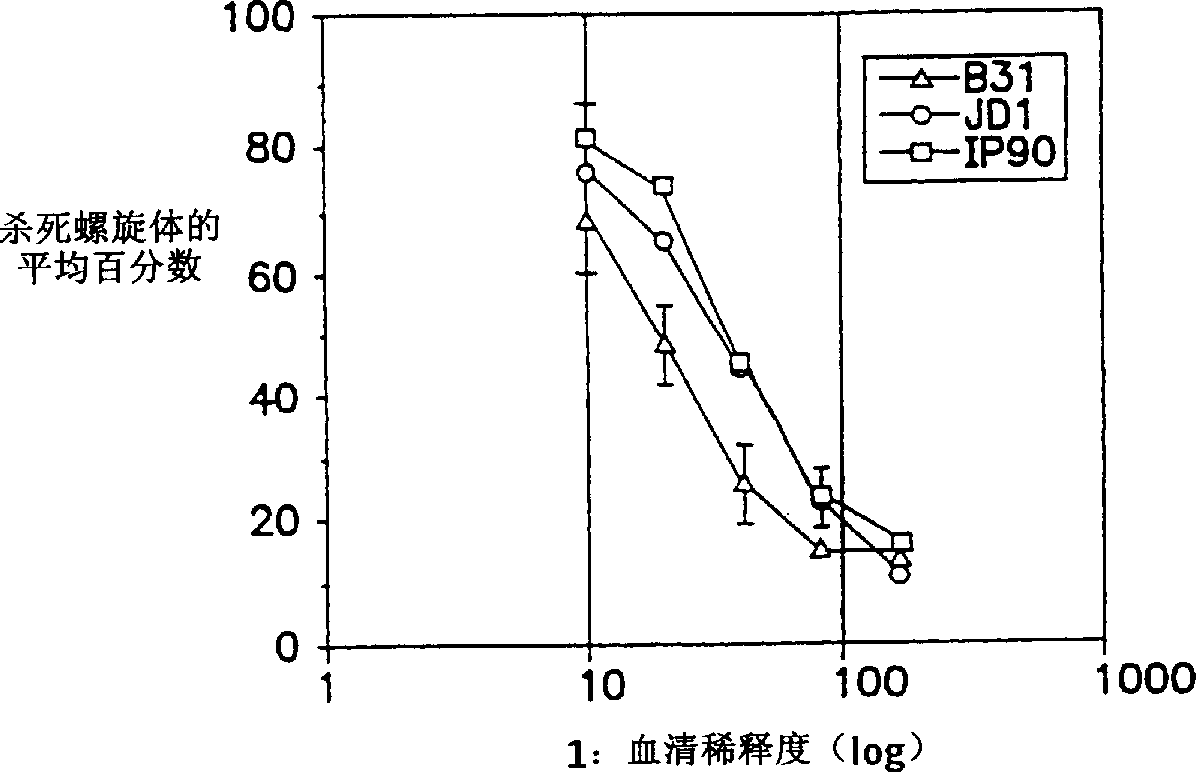
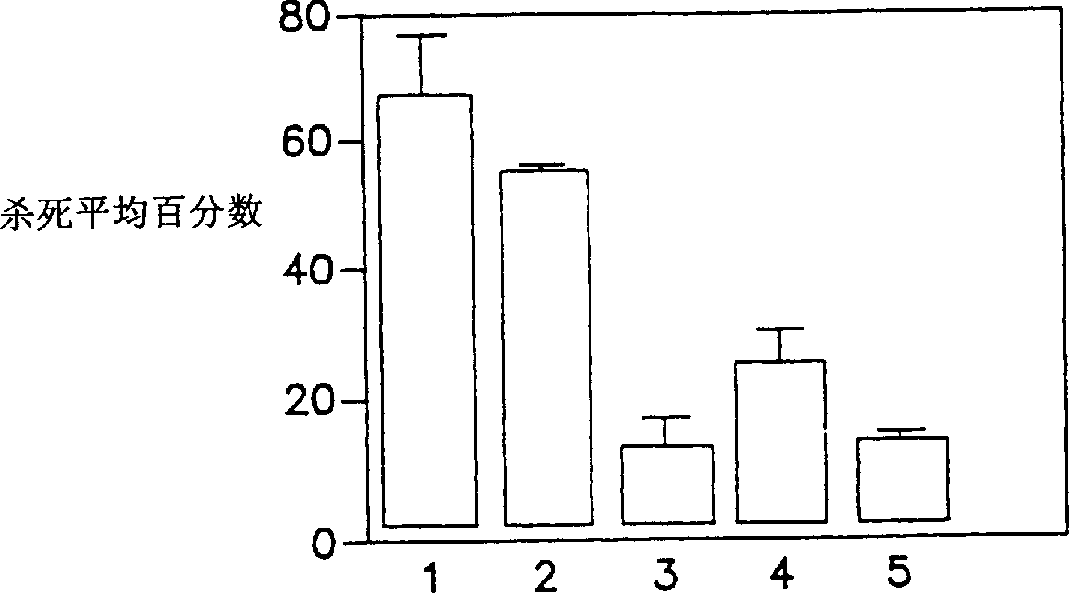
[0157] Example 3 Antibody-dependent complement-mediated killing of JD1, B31 and IP90 spirochetes
[0158] Tick-inoculated monkey sera were used in the ADCK assay as described below. Thaw frozen B. burgdorferi samples rapidly at 37°C and incubate until they reach mid-log phase (approximately 3 days, 1-2 x 10 7 Spirochetes / ml), centrifuged at 8000×g for 2 minutes, resuspended in BSK-H medium and fixed. ADCK assays were performed in duplicate in 96-well tissue culture plates (Costar). Dissolve a total of 5-6 x 10 in 25 µl of BSK-H medium 5 Spirochetes were added to each well containing 50 microliters of heat-inactivated (56°C, 30 minutes) serum samples (diluted 1:10 in the same medium). Before adding 25 μl of complement (normal monkey serum), in 3% CO 2 , 5% O 2 and balance N 2 Incubate the plate at 34°C for 20 minutes in the gas mixture. After 18-24 hours of incubation under the same conditions, the total number of dead (immobile) and live (motile) bacteria was quantified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com