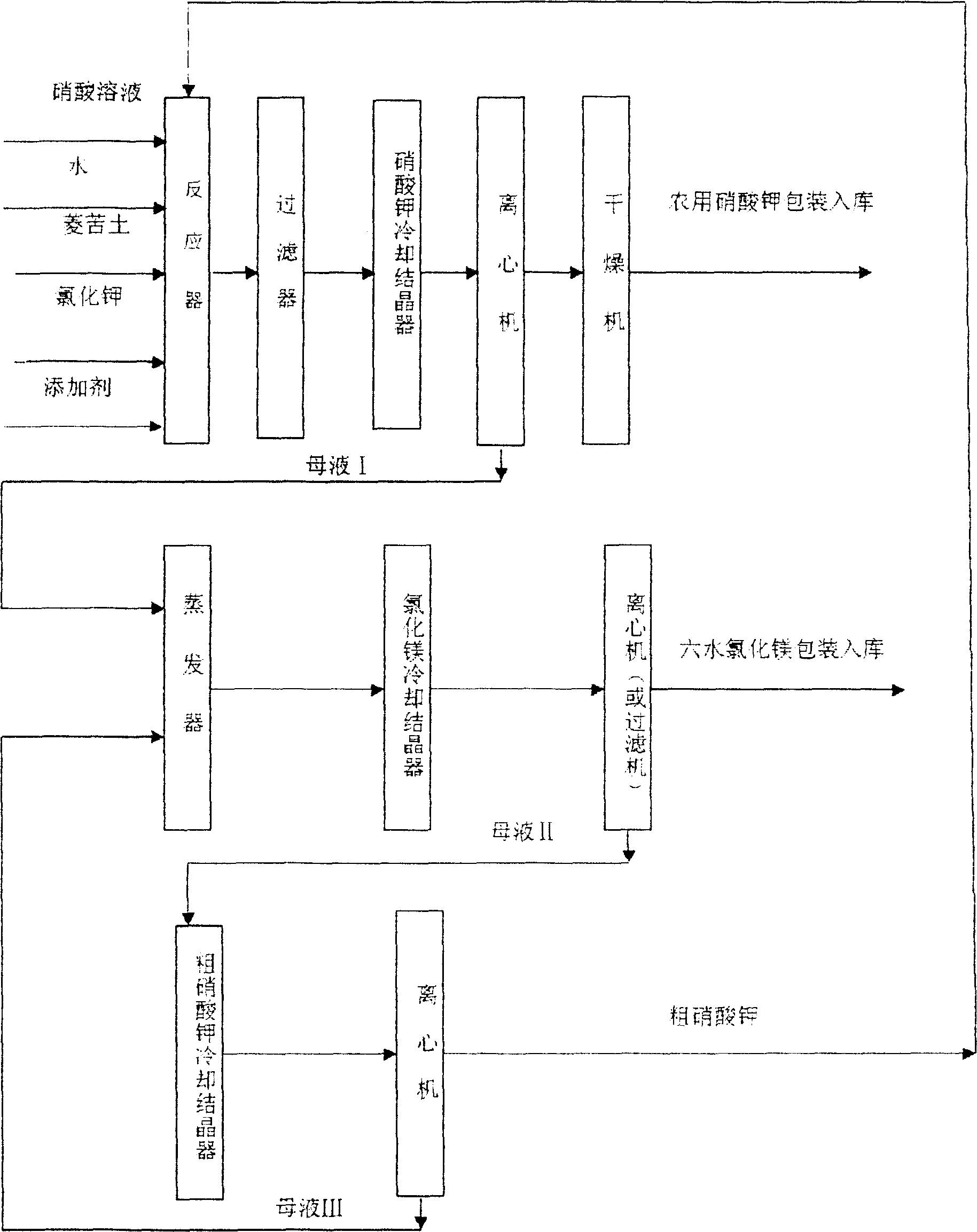
Technological process of preparing potassium nitrate and magnesium chloride
A process method and potassium nitrate technology are applied in the field of preparing potassium nitrate and by-product magnesium chloride, which can solve the problems of no recovery of magnesium chloride, low utilization rate of raw materials, environmental pollution, etc., and achieve low price, high utilization rate of raw materials, and reduced cost of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: in the nitric acid solution of 100 kilograms of 33%, gradually slowly add powdery magnesite (MgO≥85%. Free CaO≤2%, loss on ignition≤8%. Following quality is all the same), constantly stirring reaction , keep the reaction temperature at 90° C., and adjust the pH value of the solution to 6.5. Then add 40 kilograms of potassium chloride (KCl≥98%, the following mass is the same), stir and dissolve continuously, keep the reaction temperature at 115° C., add a small amount of potassium hydroxide solution and an appropriate amount of calcium chloride, and adjust the pH value of the solution to 6.5. Keep the temperature at 100°C, let the solution settle for 6 hours, filter the solution to remove impurities, then cool the solution down to 5°C, centrifuge to obtain potassium nitrate, wash the potassium nitrate crystals with a small amount of water, separate the potassium nitrate and send it to a dryer for drying , to get 50.5 kg of agricultural potassium nitrate, co...
Embodiment 2
[0036] Embodiment 2: In 90 kilograms of 45% nitric acid solution, add powdery magnesite, continuously stir and react, keep the reaction temperature at 70° C., and adjust the pH value of the solution to 7. Then add 35 kilograms of potassium chloride, stir and dissolve continuously, then add the crude potassium nitrate obtained in Example 1, stir and dissolve, keep the reaction temperature at 100° C., add a small amount of calcium hydroxide, and adjust the pH value of the solution to 6.5. Keep the temperature at 100°C, let the solution settle for 8 hours, filter the solution to remove impurities, then cool the solution down to 25°C, obtain potassium nitrate by centrifugation, wash potassium nitrate crystals with a small amount of water, separate potassium nitrate and send it to a dryer for drying , to obtain agricultural potassium nitrate, containing K 2 O44.3% (dry basis), N 13.65% (dry basis), C 10.5%, water insoluble 0.56%, moisture 0.25%. Separated mother liquor I was mixed...
Embodiment 3
[0037] Embodiment 3: In 260 kilograms of 15% nitric acid solution, add powdery magnesite, continuously stir and react, keep the reaction temperature at 90° C., and adjust the pH value of the solution to 7. Then add 40 kilograms of Potassium Chloride, continue stirring and dissolving, then add the crude potassium nitrate obtained in Example 2, stir and dissolve, keep the reaction temperature at 100° C., add a small amount of potassium hydroxide solution, and adjust the pH value of the solution to 6.5. Keep the temperature at 100°C, let the solution settle for 8 hours, filter the solution to remove impurities, then cool the solution down to 0°C, centrifuge to obtain potassium nitrate, separate the potassium nitrate and send it to a dryer for drying to obtain agricultural potassium nitrate, containing K 2 O 44.6% (dry basis), N 13.6% (dry basis), Cl 0.2%, water insoluble 0.46%, moisture 0.22%. Separated mother liquor I was mixed with mother liquor III obtained in Example 2, evapo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| loss on ignition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com