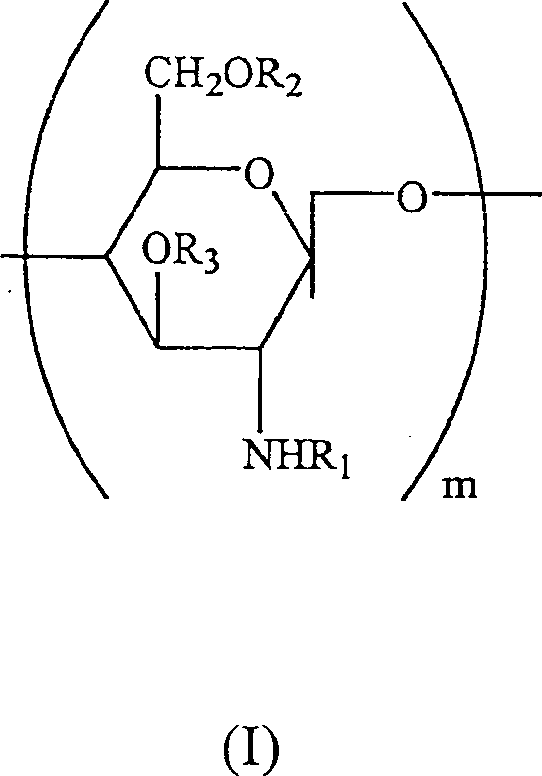
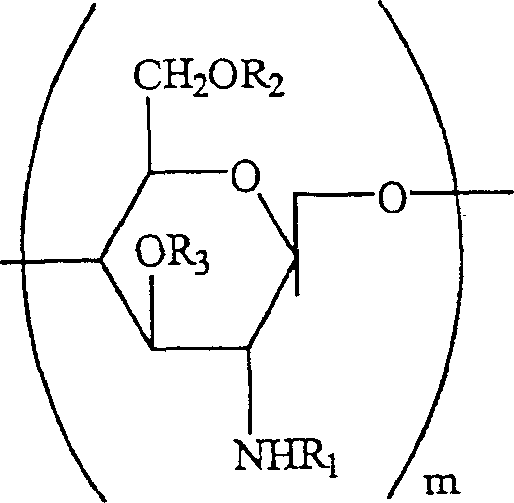
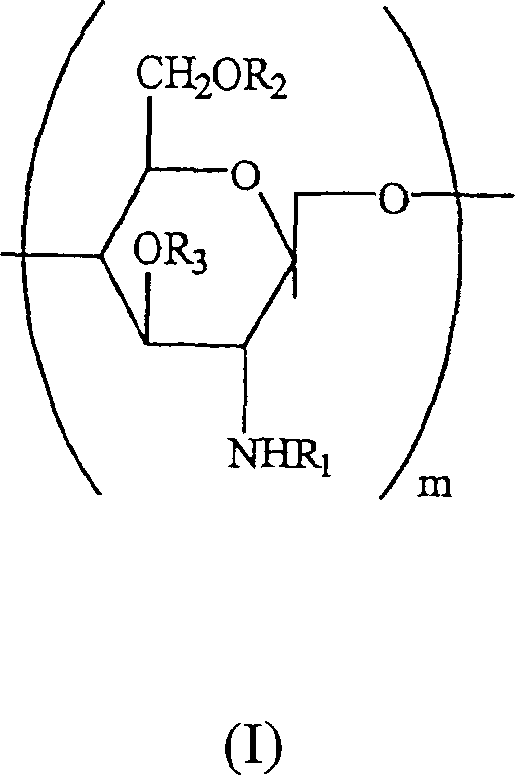
Water soluble, randomly substituted partial N-, partial O-acetylated chitosan, preserving compositions containing chitosan, and processes for making thereof
A technology of water-soluble chitosan and chitosan derivatives, applied in the field of water-soluble randomly substituted part N-, part O-acetylated chitosan, antiseptic composition containing chitosan and its preparation field, capable of Solve problems such as preventing full utilization of reaction sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] The in vitro biocompatibility study of Example 1 was based on the agar diffusion test described in USP / NF 22(87) Biological Reactivity Tests In-Vitro. In this test, on a replica agarose surface directly covered with a confluent monolayer of L-929 mouse fibroblasts, a filter plate with a 0.1 ml aliquot of Example 1 and the appropriate negative and positive control filter plates are placed . at 37°C, 5% CO 2 After culturing in medium for 24-26 hours, the culture was tested, and the results showed that Example 1 did not cause cell lysis or toxicity, thus meeting the biocompatibility requirements of USP (see Table 1-c).
[0127] The in vitro biocompatibility test result of table 1-c embodiment 1
[0128] Test / Control Item 1 Sample number Dissolution zone level 2 reactivity 1
[0129] (mm)
[0130] Example 1 1 0.0 0 None
[0131] 2 0.0 0 None
[0132] Filter Plate Control 1 0.0 0 None
[0133] 2 0.0 0 None ...
Embodiment 2
[0147] This example reveals the activity of different hydroxyalkyl chitosan solutions against E. coli in the 28 day US Preservative Efficacy Test (PET). Solutions 2a-e were prepared according to the method described in Example 1 using the following recipes.
[0148] Example formulations #2a-e:
[0149] 0.05% EDTA
[0150] 1.00% boric acid
[0151] Ultrapure water (q.s. adjusted to 100.00ml)
[0152] 0.5M sodium hydroxide (q.s. adjust pH=6.9)
[0153] Sodium chloride (q.s. adjust mOsm=300)
[0154]a: control; b=0.25% ethylene glycol chitosan (SIGMA Chemical Co.); c=0.25% hydroxypropyl chitosan (Austin Chemical Co.); d=0.25% hydroxybutyl chitosan (Austin Chemical Co. Co.); e = 0.25% di-hydroxypropyl chitosan (Technology Resource International Corporation).
[0155] The conditions for PET were the same as in Example 1, except that no reseeding was introduced on day 14. Only E. coli was selected as the screening microorganism in the test because earlier tests showed that E....
Embodiment 3
[0168] This example illustrates the effect of pH on the antimicrobial activity of ethylene glycol. The test microorganism used in Example 3 was Pseudomonas aeruginosa (ATCC No. 9027), which is a microorganism requiring attention in common contact lens-associated eye infections and infectious keratitis.
[0169] Embodiment 3 formula
[0170] Ethylene glycol chitosan (Sigma Chemical) 0.5%
[0171] Pluronic TM F68 (BASF Corporation) 0.05%
[0172] EDTA0.05%
[0173] Sodium borate decahydrate 0.08%
[0174] Boric acid 0.72%
[0175] Ultrapure water q.s adjusted to 100.00ml
[0176] Sodium hydroxide solution (0.5M) q.s adjusted to pH=6.6, 7.2 or 7.8
[0177] Sodium chloride q.s adjusted to mOsm=300±10
[0178] Table 3: Comparison of the antimicrobial activity of Example 3 against Pseudomonas aeruginosa
[0179] Pseudomonas aeruginosa Cfu after 24 hours 1,2
[0180] PH=6.6 PH=7.2 PH=7.8
[0181] 2 184 >1000
[0182] Note: 1 The inoculum concentration was 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com