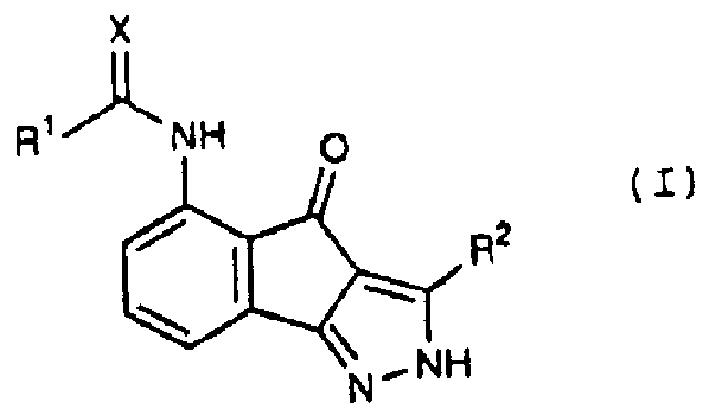
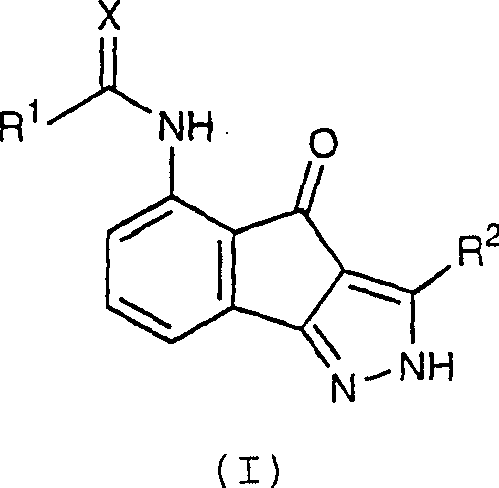
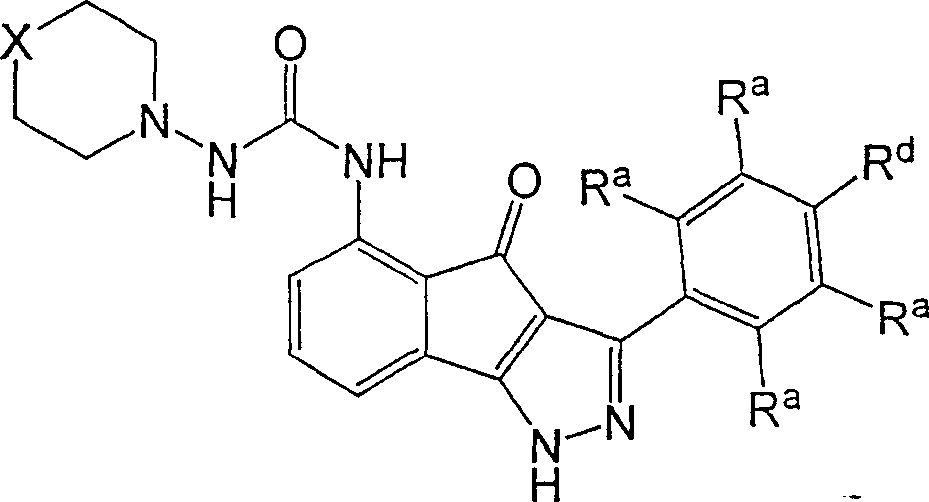
Acylsemicarbazides as cyclin dependent kinase inhibitors useful as anti-cancer and anti-proliferative agents
A compound and alkyl technology, applied in the field of 5-substituted-indeno[1, can solve problems such as unmet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0103] Preparation of 3-(4-methoxyphenyl)-5-(acetamido)indeno[1,2-c]pyrazol-4-one
[0104]
[0105] Step 1. Synthesis of 2 from dimethyl 3-nitrophthalic acid
[0106] A solution of dimethyl 3-nitrophthalic acid (25 g, 105 mmol) in methanol (100 mL) was treated with 5% Pd / C (2.5 g) and hydrogenated on a Parr shaker at 50 psi for 2 h . The solution was filtered (andoridite), the filtrate was collected, and the solvent was removed under reduced pressure. The residue was redissolved in acetic anhydride (20 mL) treated with pyridine (0.05 mL) and heated to 80°C for 1 min. The reaction was cooled and stirred at 25°C for 2 hours. The solvent was removed under reduced pressure and the residue was recrystallized from ethanol to give the product as a white solid (21 g, 79%). The melting point is 104-105°C. C 12 h 14 NO 5 The CIMS m / e calculated value is: 252.0872, the measured value is 252.0888; C 12 h 13 NO5 Anal. Calcd.: C, 57.37; H, 5.22; N, 5.58; Measured: C, 57.67; H, ...
Embodiment II
[0112] Preparation of 3-(4-methoxyphenyl)-5-(chloroacetamido)indeno[1,2-c]pyrazol-4-one
[0113]
[0114] Step 1. Synthesize 13 from 12
[0115] A suspension of 12 (1.0 g, 3.0 mmol) in methanol (10 mL) was treated with conc. HCl (1 mL), heated to reflux for 2 hours, and the reaction was cooled to collect the product as a green solid (0.7 g, 81%). Melting point 273°C, C 17 h 13 N 3 o 2 CIMS m / e calculated value: 292.1086, measured value: 292.1080; C 17 h 13 N 3 o 2 Anal. Calcd.: C, 69.85; H, 4.83; N, 14.37; Measured: 69.99; H, 4.59; N, 14.44.
[0116] Step 2. Synthesize 14 from 13
[0117] A suspension of 13 (20 mg, 0.07 mmol) in dioxane (2 mL) was washed with saturated NaHCO 3 (1 mL) in water was treated with chloroacetyl chloride (30 mL, 0.21 mmol). The reaction was heated to 50°C and stirred for 2 hours. The reaction was cooled, poured into water (2 mL), extracted with ethyl acetate (10 mL), the organic layer was separated and dried (MgSO 4 ), and the solvent...
Embodiment III
[0119] Preparation of 3-(4-methoxyphenyl)-5-(cyclopropylamido)indeno[1,2-c]pyrazol-4-one
[0120] Prepared in a similar manner as described in Example II, using cyclopropylacetyl chloride as starting material. Melting point 289°C; C 21 h 18 N 3 o 3 CIMS m / e Calculated: 360.1348, Measured: 360.1330.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com