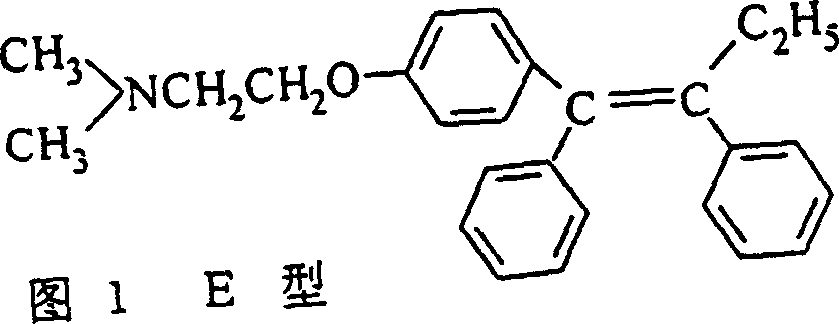
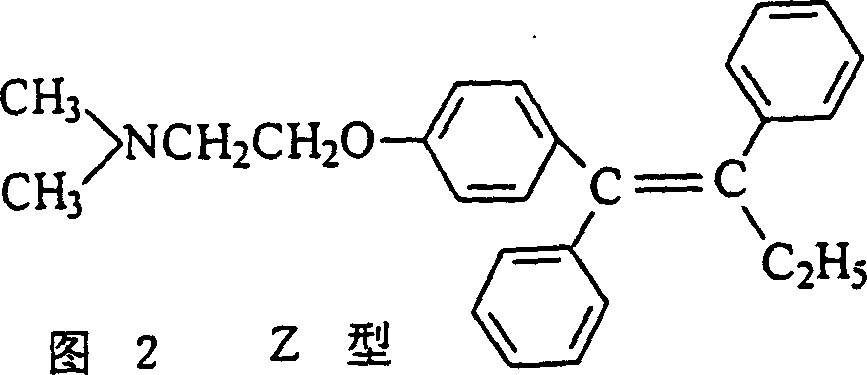
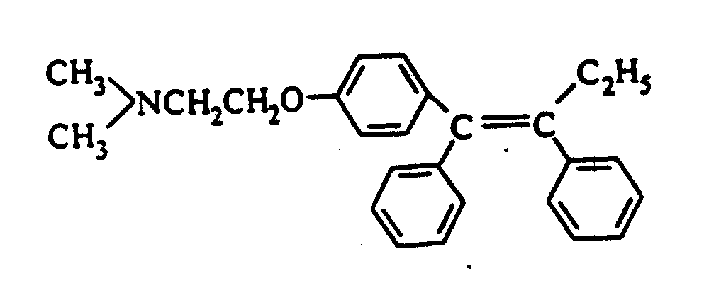
Process for preparing high purity low E type Tamoxifen citrate
A technology of tamoxifen and citrate, applied in the field of chemical pharmaceutical industry, can solve the problems of difficult production of high-purity and low-E-type tamoxifen, high-purity and low, and achieve low cost, relatively efficient separation process short process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 10 grams of tamoxifen, the content of the E-isomer is 1.8%. Add it into 200 ml of 0.05% sulfuric acid, heat and stir to dissolve; cool to room temperature, and filter to obtain tamoxifen sulfate with increased Z-isomer content.
[0032] Neutralize with alkali to obtain 8 grams of tamoxifen. Tested according to the method specified in the British Pharmacopoeia (98 edition), the content of the E-isomer is 0.02%.
Embodiment 2
[0034] 10 grams of tamoxifen, the content of the E-isomer is 2.1%. Add it into 200 ml of 1% sulfuric acid, heat and stir to dissolve; cool to room temperature, and filter to obtain tamoxifen sulfate with increased Z-isomer content.
[0035] Neutralize with alkali to obtain 6.8 g of tamoxifen. Tested according to the method specified in the British Pharmacopoeia (98 edition), the content of the E-isomer is 0.01%.
Embodiment 3
[0037] 10 grams of tamoxifen, the content of the E-isomer is 1.5%. Add it into 100 ml of 0.5% hydrochloric acid, heat and stir to dissolve; cool to room temperature, and filter to obtain tamoxifen sulfate with increased Z-isomer content.
[0038] Neutralize with alkali to obtain 7.5 g of tamoxifen. Tested according to the method specified in the British Pharmacopoeia (98 edition), the content of the E-isomer is 0.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com