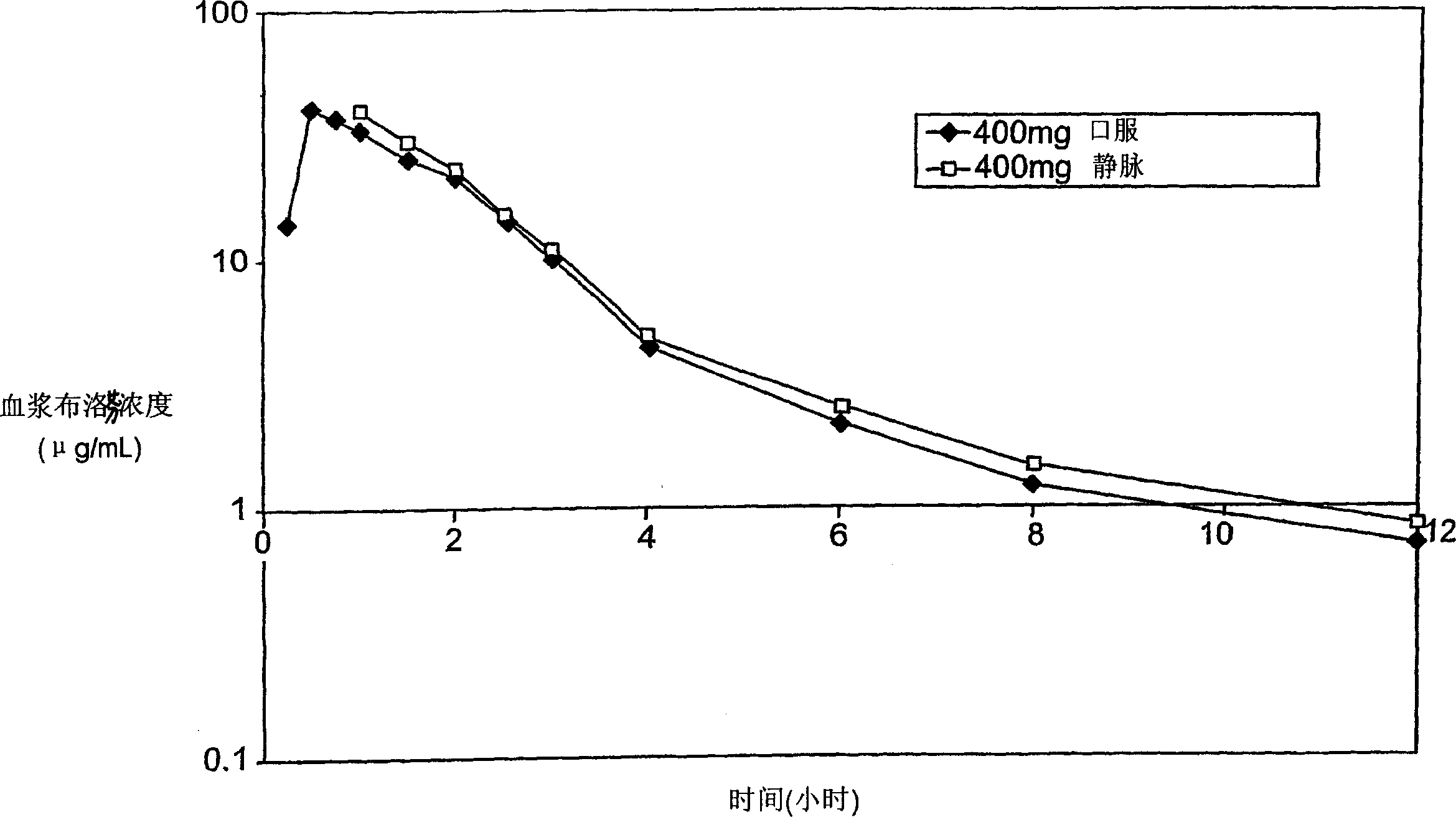
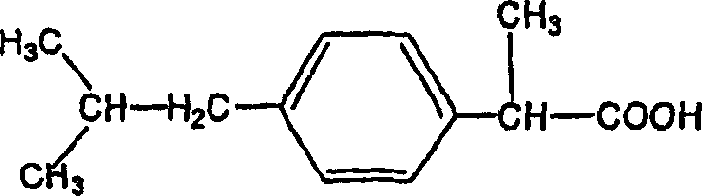
2-(4-isobutylphenyl) propionic acid medicinal composition
A kind of composition and medicine technology, applied in the pharmaceutical composition of aqueous solution, the pharmaceutical composition of amino acid is arginine, the field of manufacture of pharmaceutical composition containing arginine and ibuprofen aqueous solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 8.2 kg of arginine to approximately 80 liters of water for injection and mix until dissolved. Add 10.0 kg of ibuprofen to the arginine solution and mix until dissolved. Sufficient water was added to bring up to 100 liters to produce a 100 mg / ml solution with a molar ratio (arginine: ibuprofen) of 0.92:1. The resulting product was a clear, colorless solution that readily passed through a 0.2 micron filter. The pH of the resulting solution was about 7.4 and could be adjusted to slightly higher or lower pH as needed. The solution can be further sterilized at the end to minimize the possibility of the product not being sterilized.
Embodiment 2
[0022] Lower concentrations of ibuprofen can be prepared using lower amounts of arginine and ibuprofen. Add 41 g of arginine to approximately 80 liters of water for injection and mix until dissolved. Add 50 g of ibuprofen to the arginine solution and mix until dissolved. Sufficient water was added to bring up to 100 liters to produce a 0.5 mg / ml solution with a molar ratio (arginine:ibuprofen) of 0.92:1. The resulting product was a clear, colorless solution that readily passed through a 0.2 micron filter. The pH of the resulting solution can be adjusted to achieve the desired pH.
Embodiment 3
[0024] Lower concentrations of arginine can be used to prepare ibuprofen solutions. Add 3.8 kg of arginine to approximately 80 liters of water for injection and mix until dissolved. Add 7.5 kg of ibuprofen to the arginine solution and mix until dissolved. Sufficient water was added to bring up to 100 liters to produce a 75 mg / ml solution with a molar ratio (arginine:ibuprofen) of 0.60:1. The product can be passed through a 0.2 micron filter to give a clear colorless solution. The pH of the resulting solution can be adjusted to achieve the desired pH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com