Process for preparing high purity nickel
A high-purity, electrolytic nickel technology, applied to the improvement of process efficiency, photography technology, instruments, etc., can solve the problems of easy pollution, high production cost, and high impurity content of high-purity nickel, so as to reduce costs, prevent process pollution, and impurity content low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
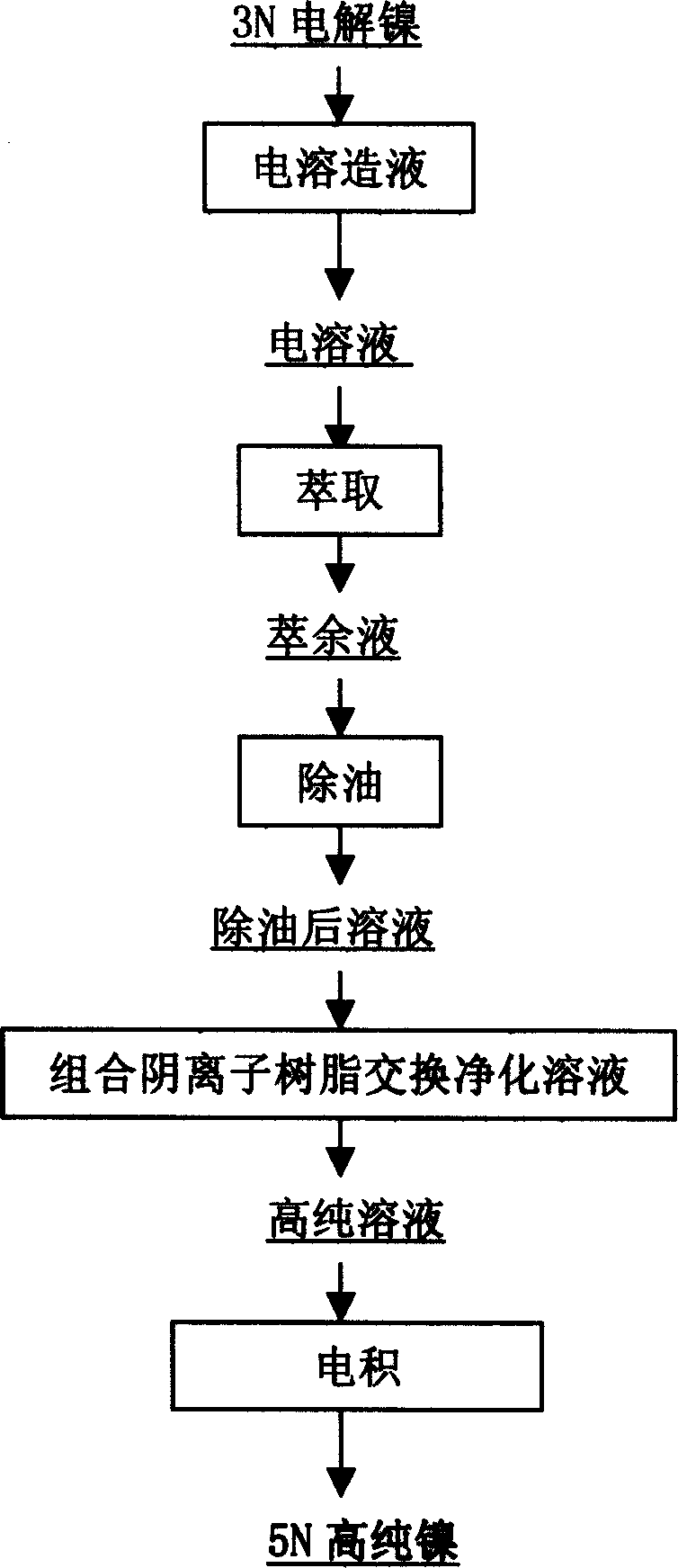
[0030] Using 3N electrolytic nickel to prepare NiCl by electrolysis in hydrochloric acid system 2 solution with a current density of 100A / m 2 , electrodissolved into the solution of H + Use 30A / m at the end of liquid production with a concentration of 1g / l 2 The current density of the liquid is produced, so that the pH of the solution is 3; the solution Cl - The concentration reaches 6mol / L, and the content of impurity elements in the solution is shown in the table:
[0031] Table 1-Content of impurity elements in the original solution Unit: g / l
[0032] No. Name Co Cu Fe Pb Zn
[0033] 1 Original solution 0.009 0.002 0.002 0.001 0.001
[0034] An anionic extractant with volume percentage of 25% tertiary amine, 45% butyl fat, and 30% sulfonated kerosene is used. After the extractant is washed with high-purity water, it is saturated with 4mol / l high-purity hydrochloric acid. Ratio 1:2, after 10 minutes of extraction balance, back-extract with pure water; after the solutio...
Embodiment 2
[0041] Using 3N electrolytic nickel to prepare NiCl by electrolysis in hydrochloric acid system 2 solution with a current density of 150A / m 2 , electrodissolved into the solution of H + Use 50A / m at the end of the fluid-making stage with a concentration of 1.5g / l 2 The current density of the liquid is produced, so that the pH of the solution is 2; the solution Cl - The concentration reaches 6mol / L, and the content of impurity elements in the solution is shown in Table 5:
[0042] Table 5-Content of impurity elements in the original solution Unit: g / l
[0043] No. Name Co Cu Fe Pb Zn
[0044] 1 Original solution 0.008 0.003 0.001 0.001 0.001
[0045] An anionic extractant with volume percentage of 40% tertiary amine, 20% butyl fat, and 40% sulfonated kerosene is used. After the extractant is washed with high-purity water, it is saturated with 4mol / l high-purity hydrochloric acid. Ratio 1:2, after 10 minutes of extraction balance, back-extract with pure water; afte...
Embodiment 3
[0052] Other conditions are the same as in Example 2, and the volume percentage of anion extractant is 20% of tertiary amine, 45% of butyl fat, and 35% of sulfonated kerosene.
[0053] The solution after ion exchange in Example 2 was used, and the components of the solution after exchange are shown in Table 8.
[0054] serial number
name
co
Cu
Fe
Pb
Zn
1
After ion exchange purification
<0.0004
0.0001
<0.0004
<0.0001
0.0001
[0055] Using this NiCl 2 Solution, insoluble anode electrowinning, electrowinning technical conditions: current density is 200A / m 2 ,, NiCl 2 The pH value of the solution is 2, and the electrodeposition temperature is 60°C. 5N high-purity nickel was prepared by insoluble anode electrowinning. High-purity nickel contains less than 0.1ppm of alkali metal elements, less than 1ppm of Fe, Co, and Cr, less than 0.1ppb of U and Th, less than 60ppm of C, and less than 100ppm of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com