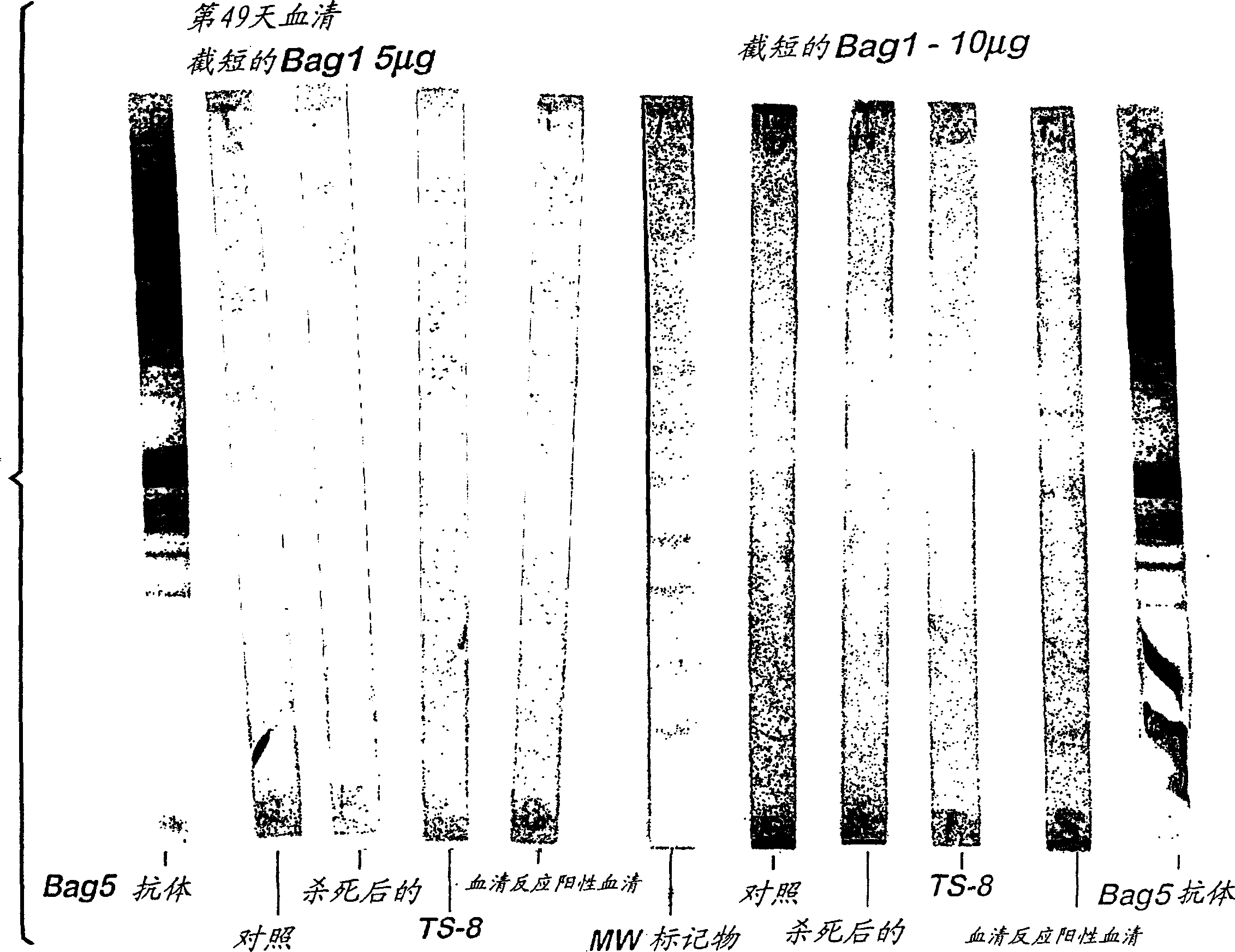
Serological assay for neospora caninum
A technology of Neospora and serum, applied in the field of serological detection, can solve the problem of not developing diagnostic tests and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Antisera from Neospora seronegative animals and Neospora inoculated animals
[0034] Serum samples from any seronegative or PCR negative animal that subsequently received a modified live, inactivated or subunit based vaccine against Neospora tachyzoites may be used. In this example, 4-month-old calves were seronegative as determined by a diagnostic ELISA test by a licensed veterinary diagnostic laboratory (Washington State Diagnostic Lab, Pullman, WA). The calves were treated with a tachyzoite-based i) modified, live, attenuated Neospora vaccine (USPTO Patent Application No. 09 / 260,414 "Live Attenuated Neospora Vaccine" at days 0 and 21, here incorporated by reference), or ii) inactivated, whole cell homogenate Neospora vaccine (USPTO Patent Application No. 09 / 138,985 "Neospora Vaccine", incorporated herein by reference) vaccination. Both vaccines are based on the tachyzoite antigen as the protective component of the vaccine. The modified, live, attenuated vaccine a...
Embodiment 2
[0036] Antisera from seropositive animals naturally infected with Neospora
[0037] Serum samples from any naturally infected, seropositive or PCR positive animal can be used. In this example, the serological status of each animal in the purchased herd was determined using a diagnostic ELISA test by a licensed veterinary diagnostic laboratory (Washington State Diagnostic Lab, Pullman, WA). Twenty-one animals reported as seropositive by this test were identified. Serum samples from each seropositive animal were collected, pooled, and aliquoted and stored frozen at -20°C until serological assay testing (see Example 4).
Embodiment 3
[0039] Bradyzoite antigens used in neospora serological tests
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com