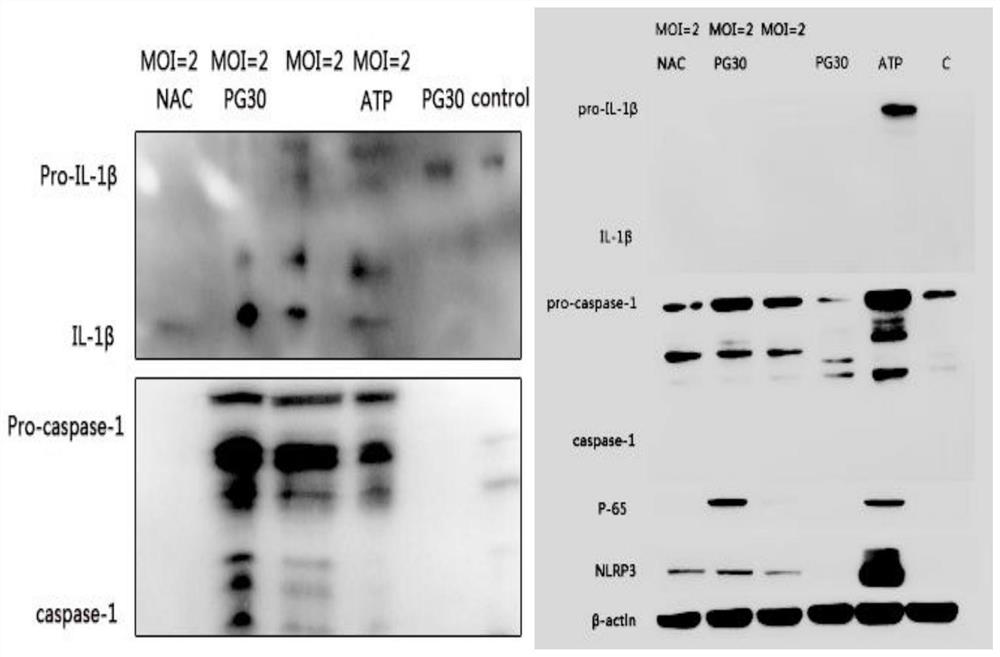
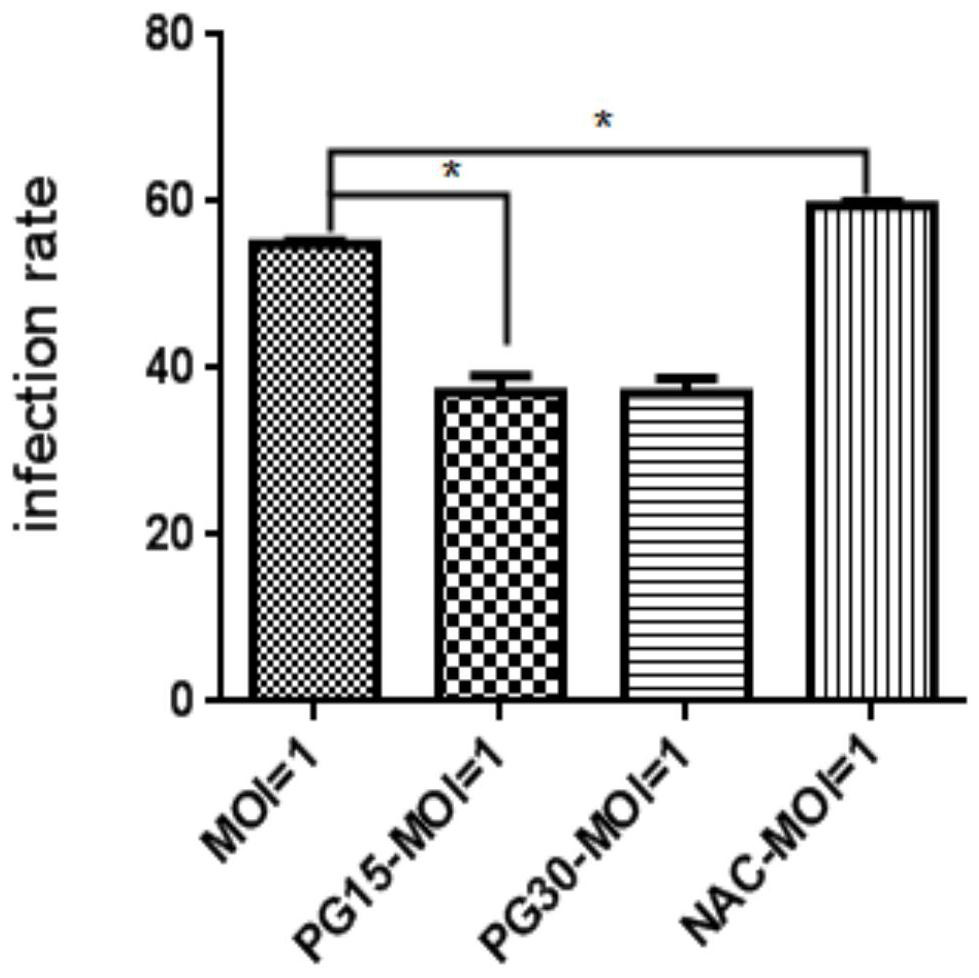
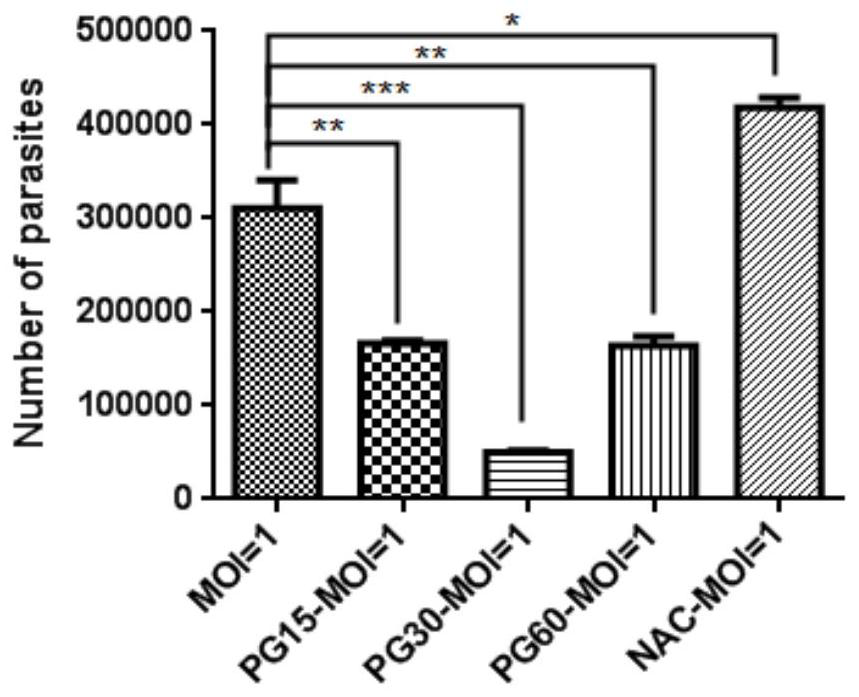
Patents
Literature
40 results about "Neospora species" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
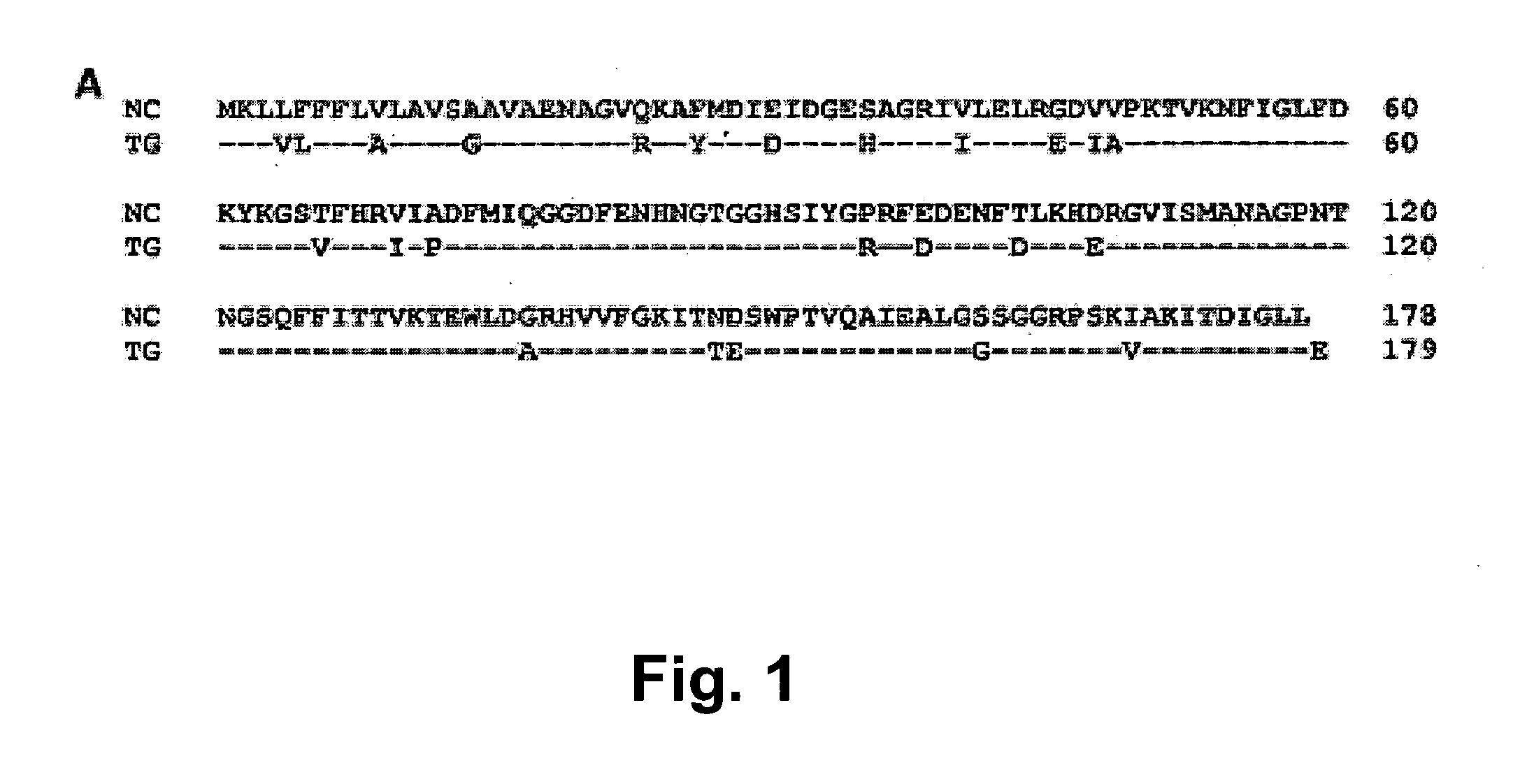
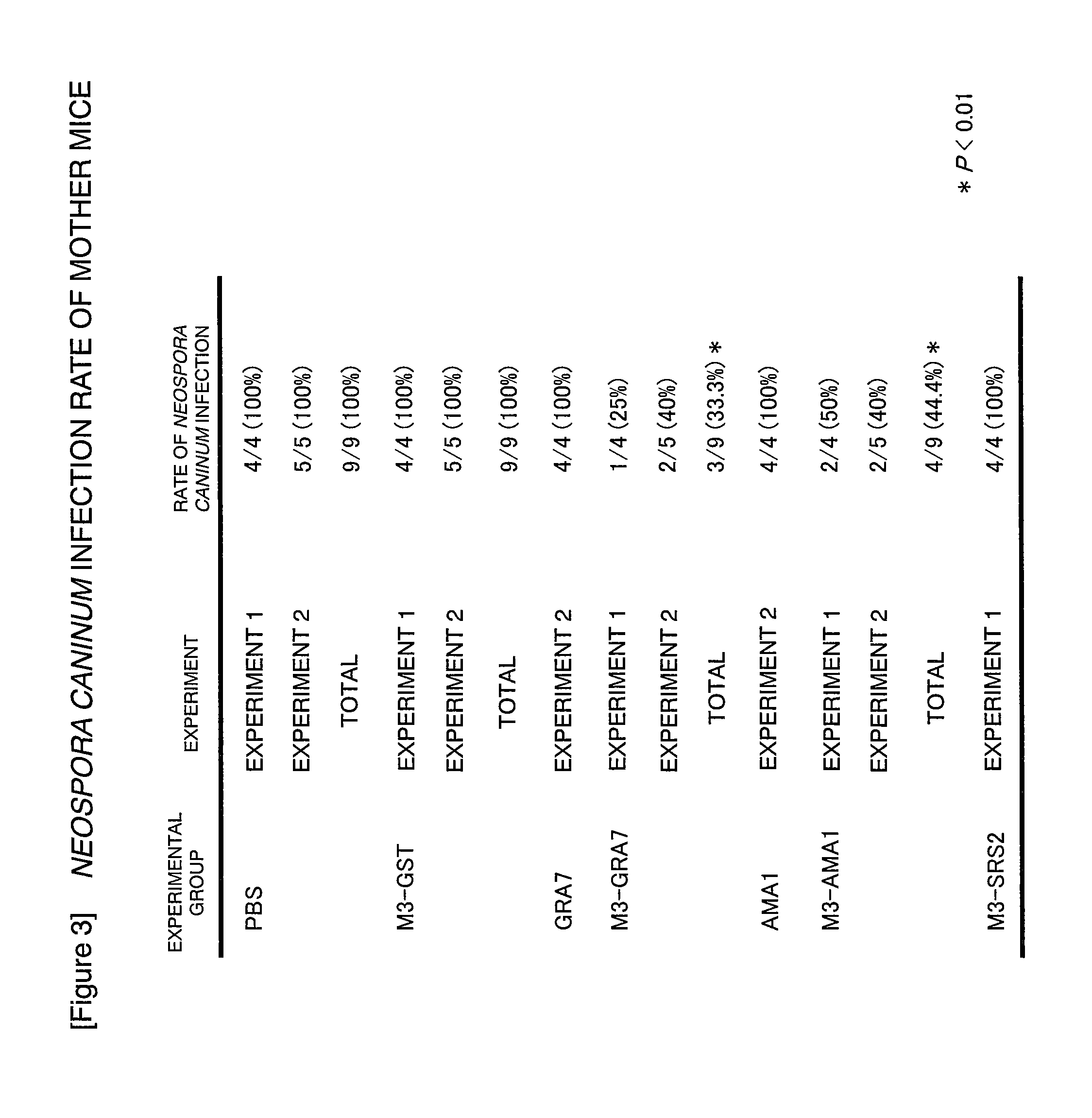
Neospora caninum is a coccidian parasite that was identified as a species in 1988. Prior to this, it was misclassified as Toxoplasma gondii due to structural similarities. The genome sequence of Neospora caninum has been determined by the Wellcome Trust Sanger Institute and the University of Liverpool.
Neosporosis recombinant enzyme-linked immunosorbent assay (rELISA) antibody assay kit
InactiveCN102455360ADemonstration effect is goodSignificant technological progressHybrid peptidesVector-based foreign material introductionAntigenEscherichia coli
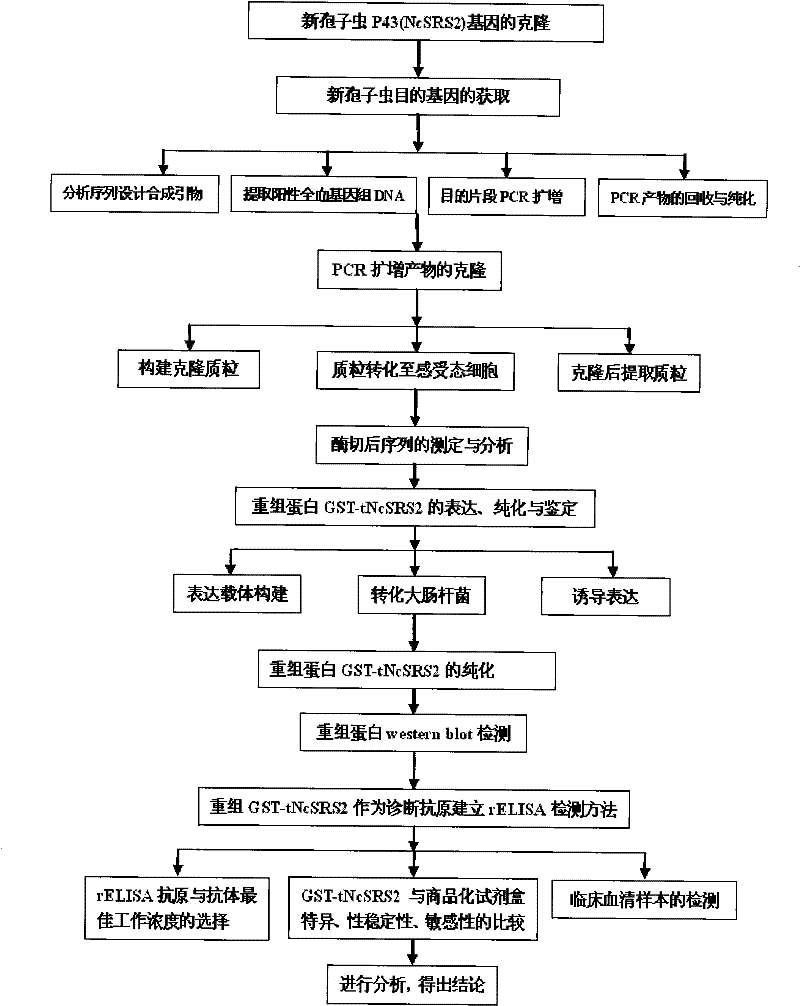
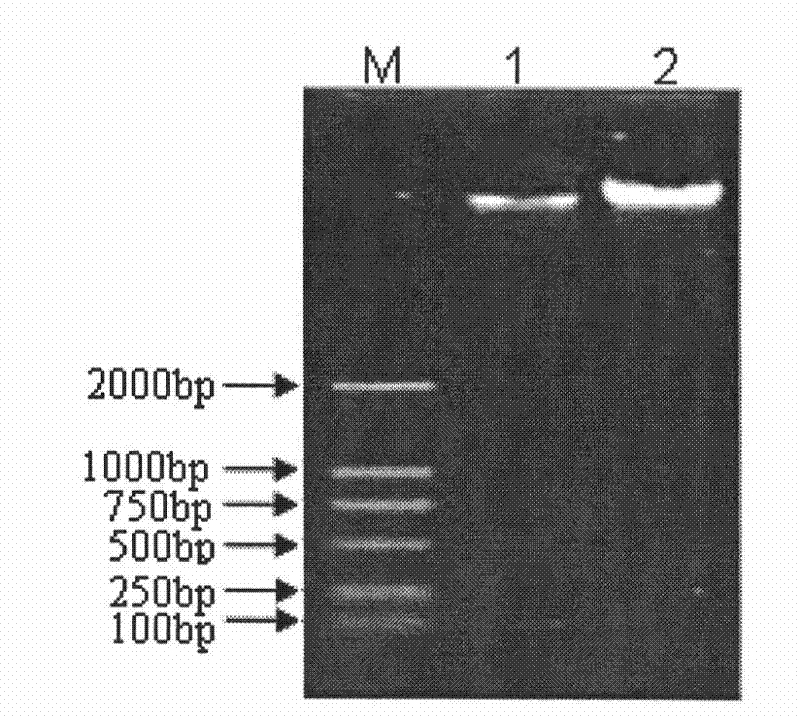
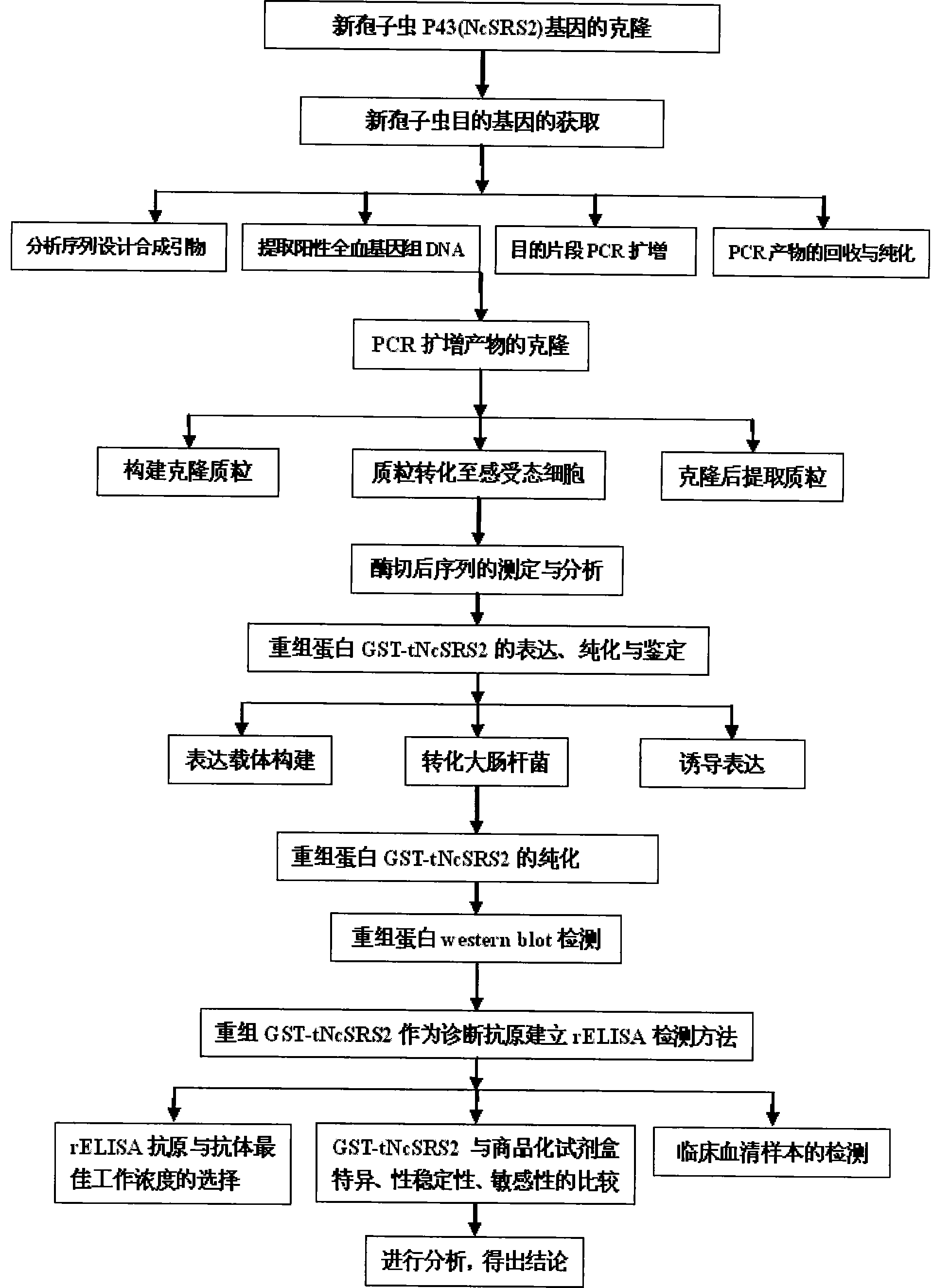
The invention provides a neosporosis rELISA antibody assay kit. The neosporosis recombinant enzyme-linked immunosorbent assay (rELISA) antibody assay kit is characterized in that designing a primer set according to a neospora caninum NcSRS2 gene of the GenBank, introducing the primer set to an enzyme digestion site, removing a signal peptide zone of an NcSRS2 protein, extracting a DNA from Xinjiang cow positive serum, carrying out polymerase chain reaction (PCR) amplification of the DNA to obtain a tNcSRS2 genetic fragment having length of 975bp, cloning the tNcSRS2 genetic fragment into a pMD-18-T carrier, carrying out enzyme digestion and sequencing, further directionally connecting the desired fragment obtained by the previous step to a pGEX-4T-2 expression carrier, transferring the pGEX-4T-2 expression carrier with the desired fragment into escherichia coli, carrying out inducible expression and purification, wherein expressed fusion protein molecular weight is about 62kDa, and carrying out ELISA antigen coating by the expressed fusion protein to obtain the neosporosis rELISA antibody assay kit. A use method of the neosporosis rELISA antibody assay kit comprises the following steps that a coated enzyme label reaction plate is taken out, is subjected to first washing, is enclosed, is subjected to second washing, is added with a sample needing to be detected, is subjected to third washing, is added with a secondary antibody, is subjected to fourth washing, and undergoes a color reaction; and when the color reaction is finished, an OD450 / 630 value of the coated enzyme label reaction plate is read and a result is determined. Compared with a commercial ELISA assay kit, the neosporosis rELISA antibody assay kit has a higher coincidence rate above 95% The neosporosis rELISA antibody assay kit also is suitable for the ELISA of a neospora caninum antibody in ruminant serum and blood plasma.
Owner:XINJIANG AGRI UNIV
Polynucleotide molecules encoding Neospora proteins
The present invention provides isolated polynucleotide molecules comprising nucleotide sequences encoding GRA1, GRA2, SAG1, MIC1 and MAG1 proteins from Neospora caninum, as well as recombinant vectors, transformed host cells, and recombinantly-expressed proteins. The present invention further provides a polynucleotide molecule comprising the nucleotide sequence of the bidirectional GRA1 / MAG1 promoter of N. caninum. The present invention further provides genetic constructs based on the polynucleotide molecules of the present invention that are useful in preparing modified strains of Neospora cells for use in vaccines against neosporosis.
Owner:PFIZER INC +1
Novel Neospora caninum Vaccine
InactiveUS20090208519A1Reduce severityPrevent and to decrease severityProtozoa antigen ingredientsViral antigen ingredientsBALB/cBuprestis novemmaculata
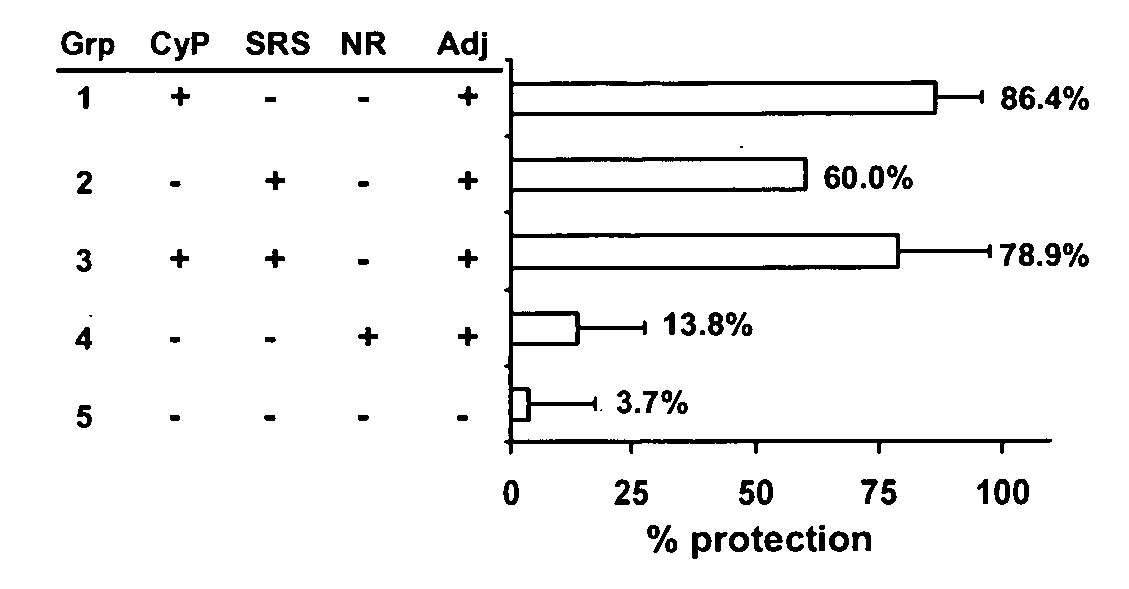
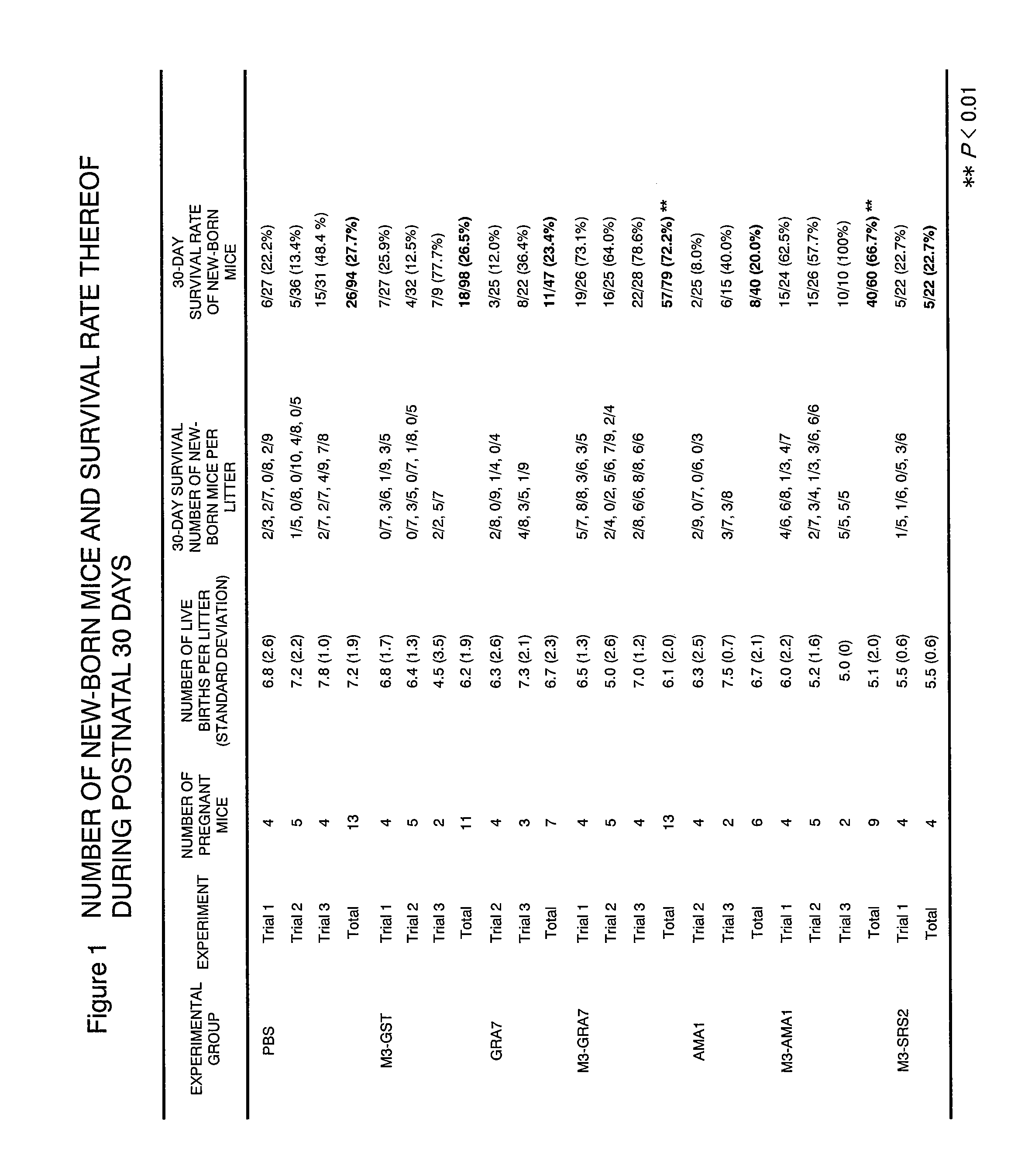
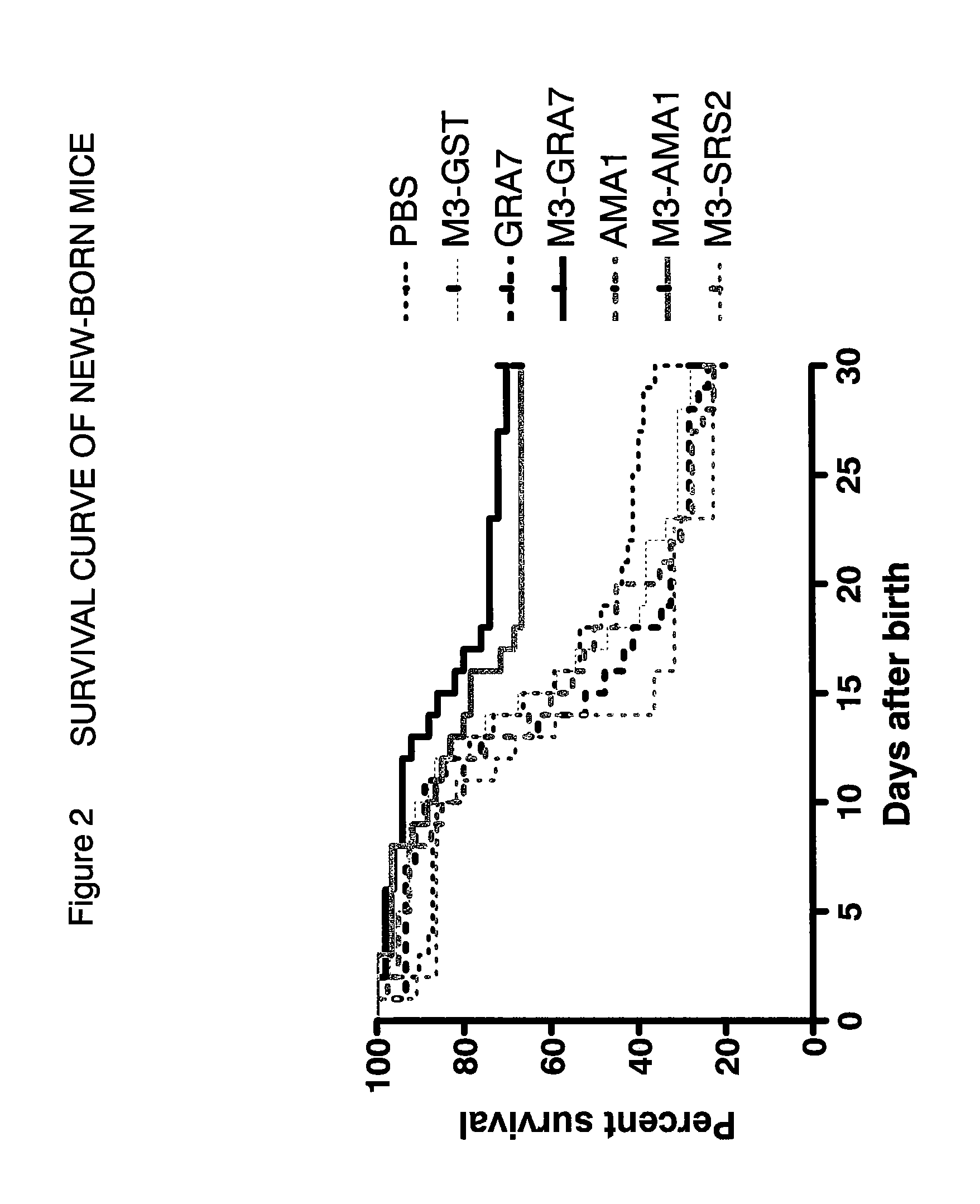
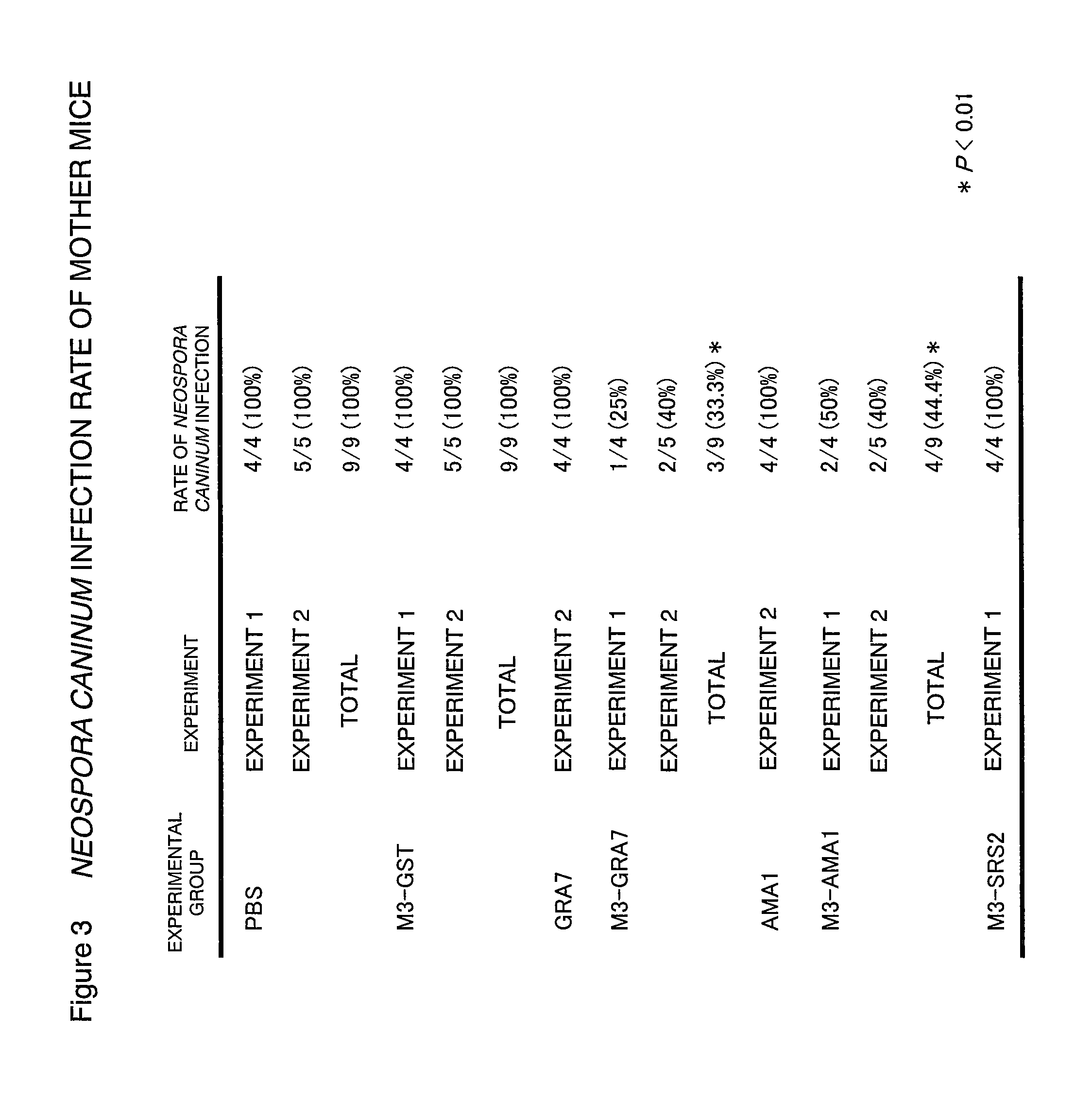
Neospora caninum is the causal agent of bovine neosporosis which results in high levels of abortion. The present study determined the protective efficacy of two Neospora antigens—Neospora cyclophilin (NcCyP) and NcSRS2. The ability of native NcCyP to upregulate mouse IFNγ was also confirmed in this study. Recombinant NcCyP or NcSRS2 were tested either alone or in combination and formulated with adjuvant ImmuMax-SR and CpG. Female BALB / c mice (n=15) of 10-12 weeks of age were immunized s.c. twice in a 2-week interval with vaccines containing either NcCyP alone, NcSRS2 alone, NcCyP plus NcSRS2, or non-recombinant bacterial antigen (NR) in 2 separate trials. All mice were challenge-infected 3 weeks following the booster immunization and necropsied 3 weeks after the challenge infection. Brain and serum were collected and Nc-specific DNA sequence in brain tissue and antibodies in serum were analyzed by PCR or ELISA / Western blotting. Results showed that mice vaccinated with rNcCyP, rNcSRS2, or both rNcCyP and rNcSRS2 responded with high levels of NcCyP or NcSRS2 specific antibodies. Overall, mice received vaccines formulated with either rNcCyP or rNcCyP and rNcSRS2 had a higher (p<0.01) percent protection when compared to the mock- or non-vaccinated mice. The groups immunized with rNcSRS2 alone exhibited slightly lower levels of protection, which was higher (p<0.05) than that of the non-vaccinated group but did not differ (p=0.06) from that of the mock-vaccinated group. The results of the present study indicate that NcCyP is a highly efficacious vaccine candidate which may be useful in protection against Neospora infection.
Owner:UNITED STATES OF AMERICA
Neospora caninum NcMIC26 antigen and application thereof
InactiveCN110183527AEasy to operateIncreased sensitivityAntiparasitic agentsFermentationPositive sampleNeospora species
The invention discloses a neospora caninum NcMIC26 antigen and an application thereof. Firstly, the invention discloses neospora caninum NcMIC26 antigen. The amino acid sequence of the antigen is as shown in SEQID NO.1. The invention further discloses an application of recombinant protein of the antigen to construction of a neospora caninum indirect ELISA diagnosis reagent kit. Through screening,a new diagnosis antigen suitable for neospora caninum is obtained, an indirect ELISA reagent kit is established by using the recombinant protein of the antigen as a coating antigen, the operation is simple, quick and efficient, the specificity is good, the sensibility is high, the repeatability is good, the neospora caninum NcMIC26 antigen can be used for detecting neospora caninum infection situation of cattle and can detect positive samples which cannot be detected by common NcSRS2 protein, more accurate detection data can be obtained, and the neospora caninum NcMIC26 antigen has important significance in preventing and treating the neospora caninum.
Owner:CHINA AGRI UNIV
Neospora caninum isolate
Owner:UNIV OF TECH SYDNEY
Vaccine Preparation for Neospora Caninum Infection
InactiveUS20110250265A1High prophylactic effectReduce riskPeptide/protein ingredientsAntiparasitic agentsAntigenSpore
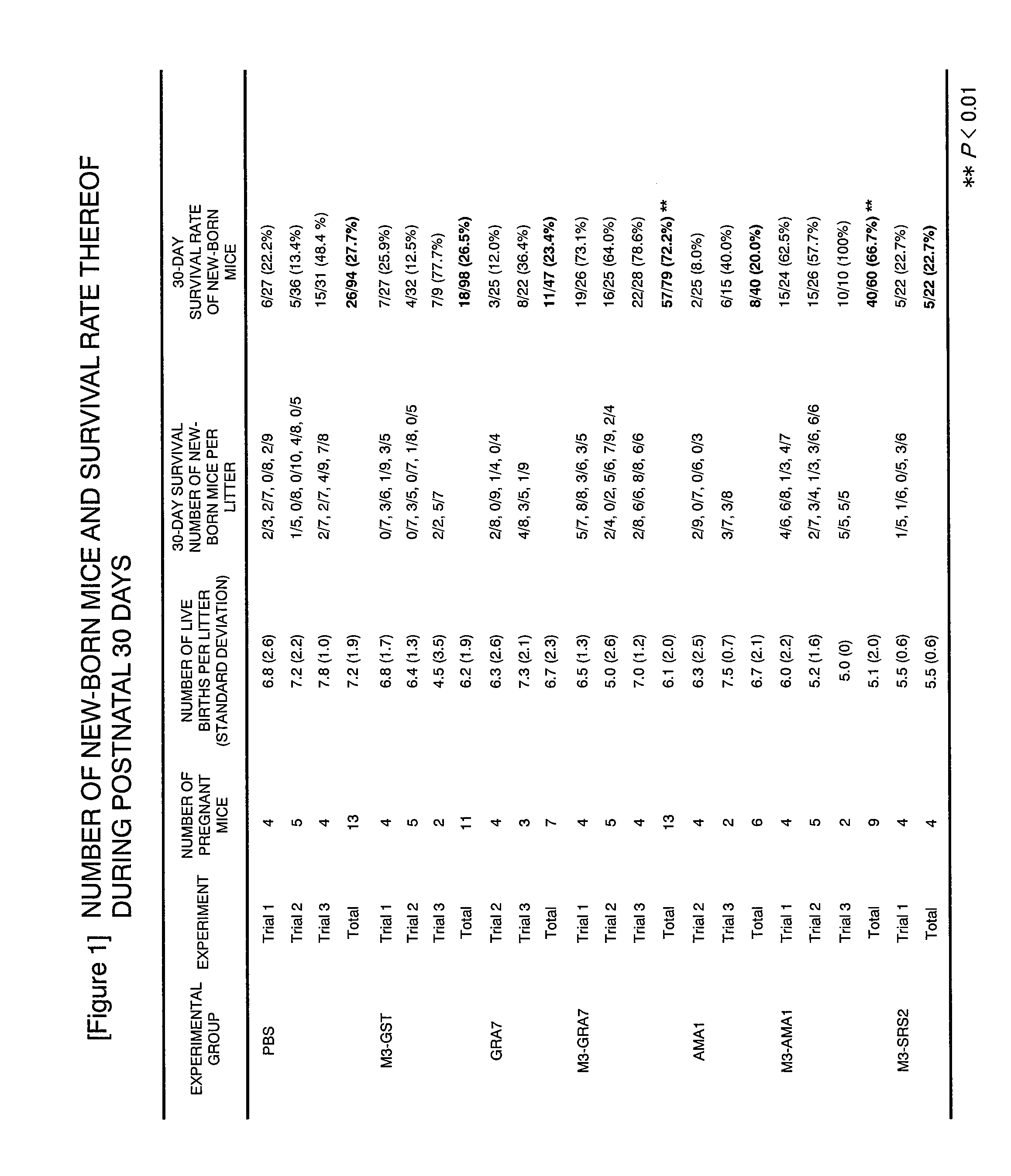
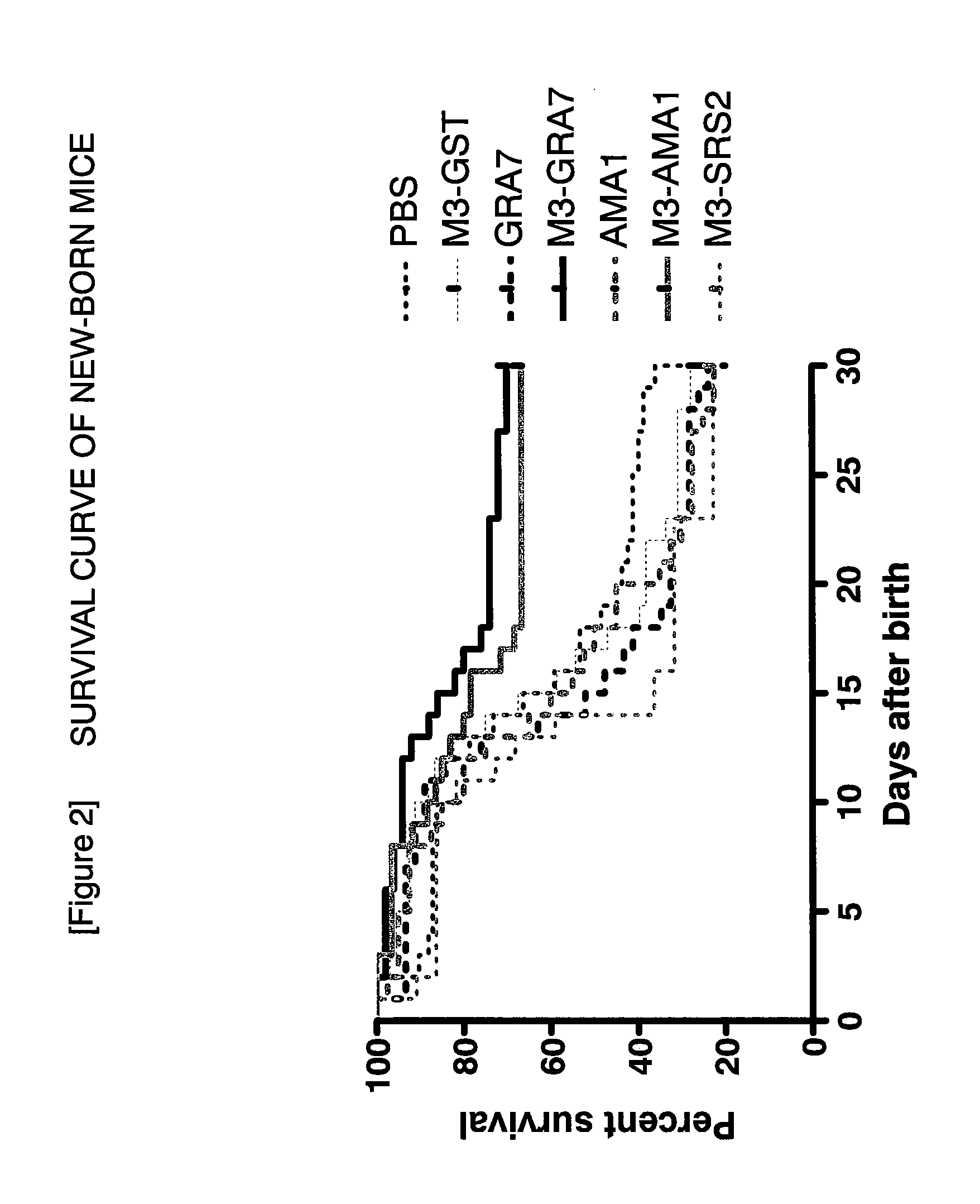
A vaccine preparation characterized in that Neospora caninum-derived dense granule protein 7 or apical membrane antigen 1 or an immunologically active variant or derivative thereof is included in liposomes each having an oligosaccharide capable of binding to a carbohydrate recognition molecule on the surface of antigen-presenting cells on the surface of the liposome.
Owner:OBIHIRO UNIVERSITY OF AGRICULTURE AND VETERINARY MEDICINE +1
Novel method for in vitro culturing and staining Neospora caninum tachyzoites
InactiveCN102533556AThe cultivation method is simpleQuick observationProtozoaMicrobiological testing/measurementNeospora speciesMicroscopic observation
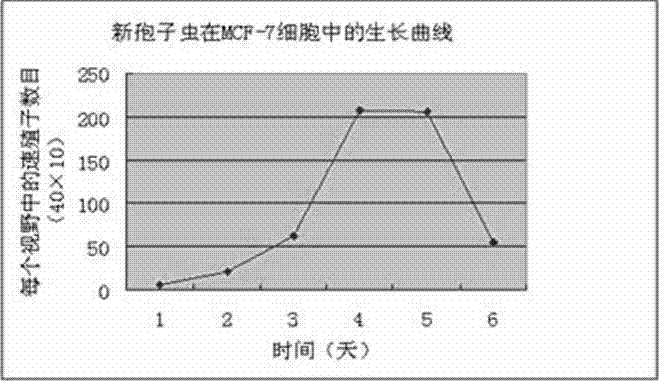
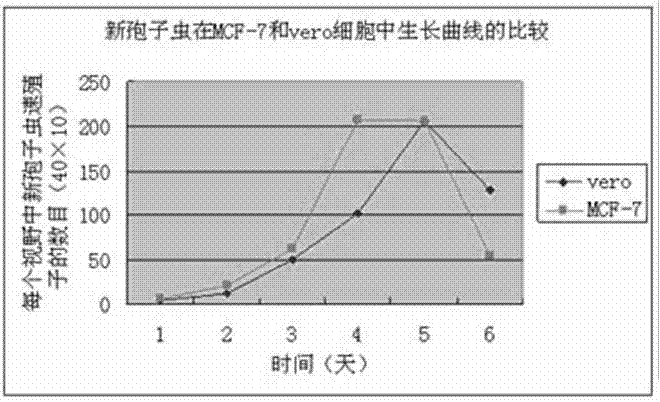
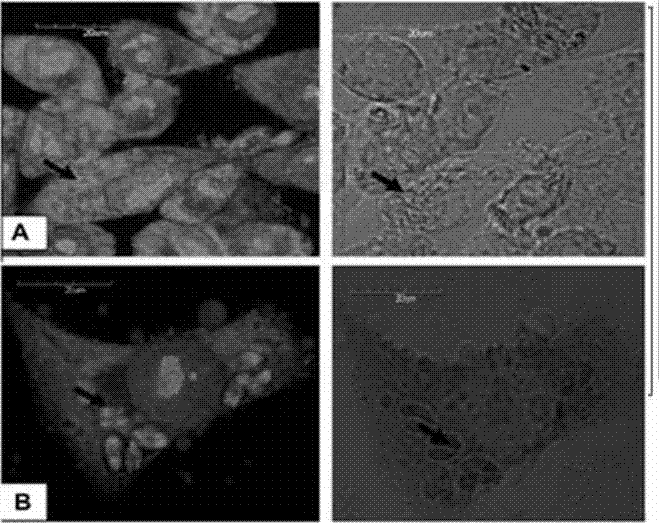
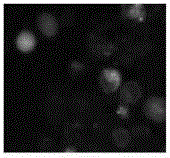
The invention relates to a novel method for in vitro culturing and staining Neospora caninum tachyzoites, which is characterized in that the concrete culturing steps are as follows: culturing MCF-7 breast cancer cells using a conventional method, inoculating in 1ml Neospora caninum at an inoculum size of 10<4> / ml after the cells grow to form a monolayer of cells, culturing with RPIM-1640 medium containing 2% fetal bovine serum, and observing the number of extracellular Neospora caninum tachyzoites with an inverted microscope everyday; and the concrete staining steps are as follows: fixing Neospora caninum tachyzoites with paraformaldehyde, staining with acridine orange, and observing and taking photos with a common fluorescence microscopy. The culturing method of the invention is simple and practical, and Neospora caninum tachyzoites can be rapidly cultured. In addition, after acridine orange staining, intracellular parasites can be rapidly, visually and clearly observed with the common fluorescence microscopy.
Owner:JILIN UNIV
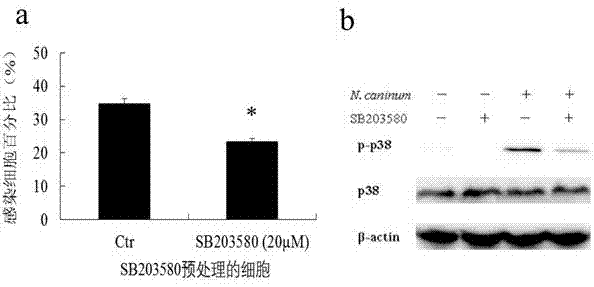
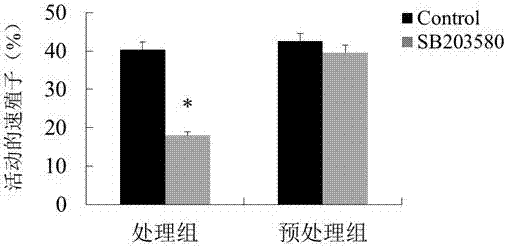
Novel anti-neosporosis drug
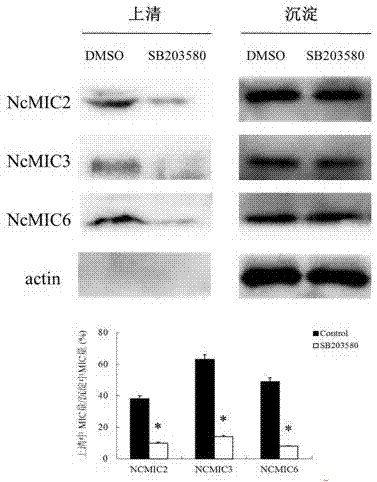
The invention provides a novel anti-neosporosis drug, provides a new medical application of SB203580 and finds a chemical drug which can remarkably inhibit cell infection of neosporosis and infection of brain tissue of a mouse model and plays a blocking function by acting on host cells and parasites simultaneously. A novel candidate drug and a novel candidate drug action target p38 MAPK are provided for treatment of neosporosis, and a foundation is laid for future drugs for intervening the neosporosis.
Owner:JILIN UNIV
Serological assay for neospora caninum
The present invention relates to the serological detection of animals inoculated with Neospora by reacting the test serum with a protein reactive with Neospora. The protein may be a full-length natural or recombinant Neospora or T. gondii bradyzoite fusion protein or a truncated fusion protein or a fragment thereof. The protein can be used in any of a number of assays including enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), Western blot and other suitable forms of immunoassay.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
RAA primer, probe and detection method for detecting pasteurella multocida diseases
PendingCN111621580ARealize detectionQuick checkMicrobiological testing/measurementMicroorganism based processesEscherichia coliDisease
The invention discloses a primer, probe and detection method for detecting pasteurella multocida by an RAA fluorescence method. The primer and the probe are suitable for detection by the RAA fluorescence method, can accurately detect pasteurella multocida plasmids and positive samples, and do not have cross reactions with mycoplasma, streptococcus, escherichia coli, eperythrozoon, neospora caninum, toxoplasma gondii and healthy cow blood, and the specificity reaches 100%. The detection method is quick, and high flux is easy to realize, besides, the detection time is reduced, and the detectioncost is reduced. A method for quickly detecting pasteurella multocida DNA based on the RAA fluorescence method, provided by the invention, is high in sensitivity, and the reaction detection sensitivity reaches 10copies / [mu] L.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
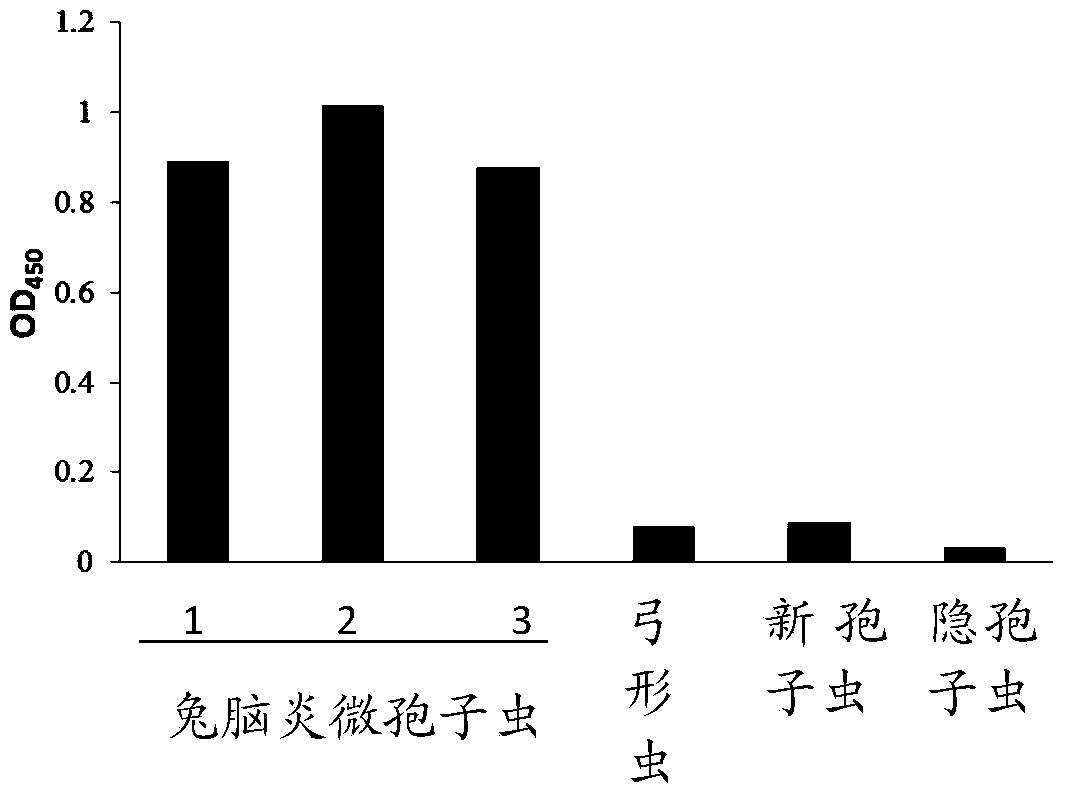
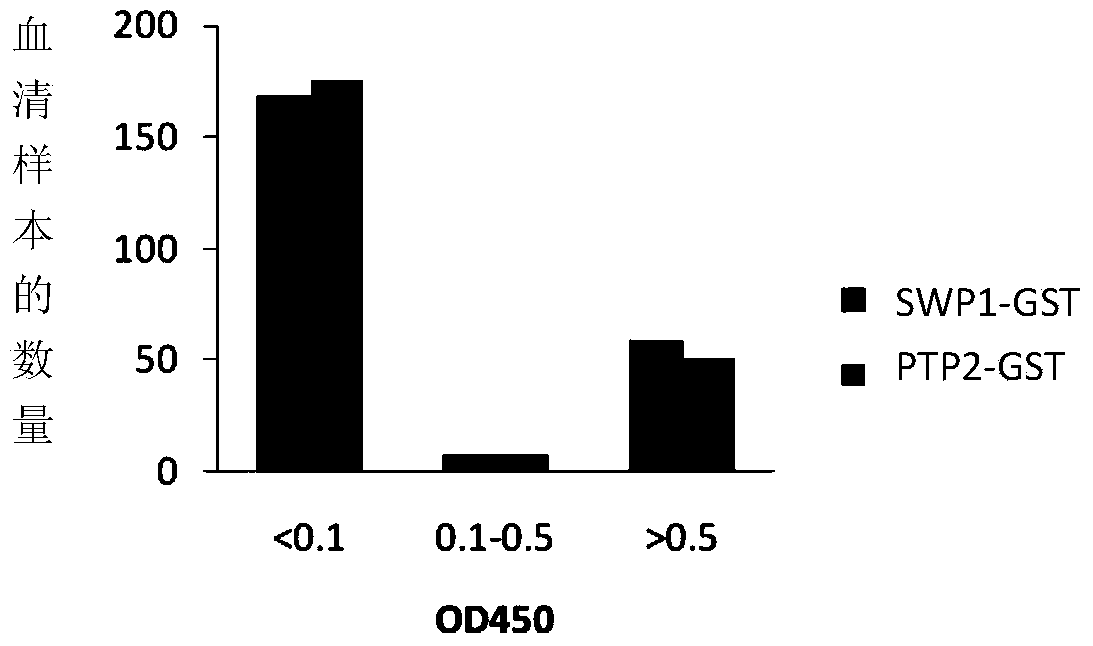
Applications of rabbit encephalitozoon cuniculi spore wall protein SWP1 to preparation of reagent for diagnosing or detecting rabbit encephalitozoon cuniculi infection
InactiveCN103728458AIncreased sensitivityBiological testingFermentationCryptosporidium infectionSparganosis
The invention discloses application of rabbit encephalitozoon cuniculi spore wall protein SWP1 to preparation of a reagent for diagnosing or detecting rabbit encephalitozoon cuniculi infection. A specific primer is designed according to the gene sequence of the rabbit encephalitozoon cuniculi spore wall protein SWP1, published by a database of NCBI (National Center of Biotechnology Information), and is amplified in a fox kidney sample infected with encephalitozoon cuniculi to obtain the SWP1nucleotide sequence shown as SEQ ID NO.2. The fused-expressed SWP1-GST is used for the detection of the sample, the result shows that the fused-expressed SWP1-GST protein has good specificity on the detection of fox serum infected with rabbit encephalitozoo cuniculi, and has no cross reaction with positive serum infected with toxoplasma gondii, neospora caninum and cryptosporidium. In addition, the research result shows that the sensitivity of the SWP1 protein, which is taken as a detection antigen, is obviously higher than that of the PTP2 (Protein-tyrosine-phosphatase 2) protein as a detection antigen. Therefore, the method for diagnosing or detecting the rabbit encephalitozoonosis cuniculi is high in sensitivity, and the diagnosis and prevention of the rabbit encephalitozoonosis cuniculi are further promoted.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +3
Neospora vaccines
A Neospora caninum vaccine comprising tissue culture grown Neospora and methods of making and using said vaccines. Neospora caninum vaccines described include those containing whole Neospora tachyzoites, extracts of Neospora tachyzoites and protective antigen subunits of Neospora tachyzoites. The vaccines of this invention may be in a liquid or lyophilized form.
Owner:INTERVET INT BV
Neospora caninum specific nest-polymerase chain reaction (PCR) test kit and preparation method
InactiveCN102888412AMicrobiological testing/measurementFermentationInapparent InfectionNeospora species
The invention provides a neospora caninum specific nest-polymerase chain reaction (PCR), which is used for testing tachyzoite, tissue cyst and oocyst infected with neospora caninum in different animals. The invention also discloses a preparation method of the nest-PCR test kit. The nest-PCR is used for amplifying some specific molecular fragment of a gene of a neospora caninum for diagnosing neosporosis. The invention relates to neospora caninum and a neospora caninum specific gene SEQNo.1 which is screened from an ultramicro-structure suppressed subtractive hybridization library. The gene is the specific to neospora caninum, so amplification of fragments of SEQNo.1 gene in animal tissue and dung by the nest-PCR can be used for molecule detection of neospora caninum and diagnosing acute, chronic and recessive infection with neospora caninum.
Owner:JILIN UNIV
Neospora vaccines
InactiveUS7462359B2Bacterial antigen ingredientsProtozoa antigen ingredientsProtective antigenAntigen
A Neospora caninum vaccine comprising tissue culture grown Neospora and methods of making and using said vaccines. Neospora caninum vaccines described include those containing whole Neospora tachyzoites, extracts of Neospora tachyzoites and protective antigen subunits of Neospora tachyzoites. The vaccines of this invention may be in a liquid or lyophilized form.
Owner:INTERVET INT BV
Modification method and application of neospora caninum surface protein SRS2 gene
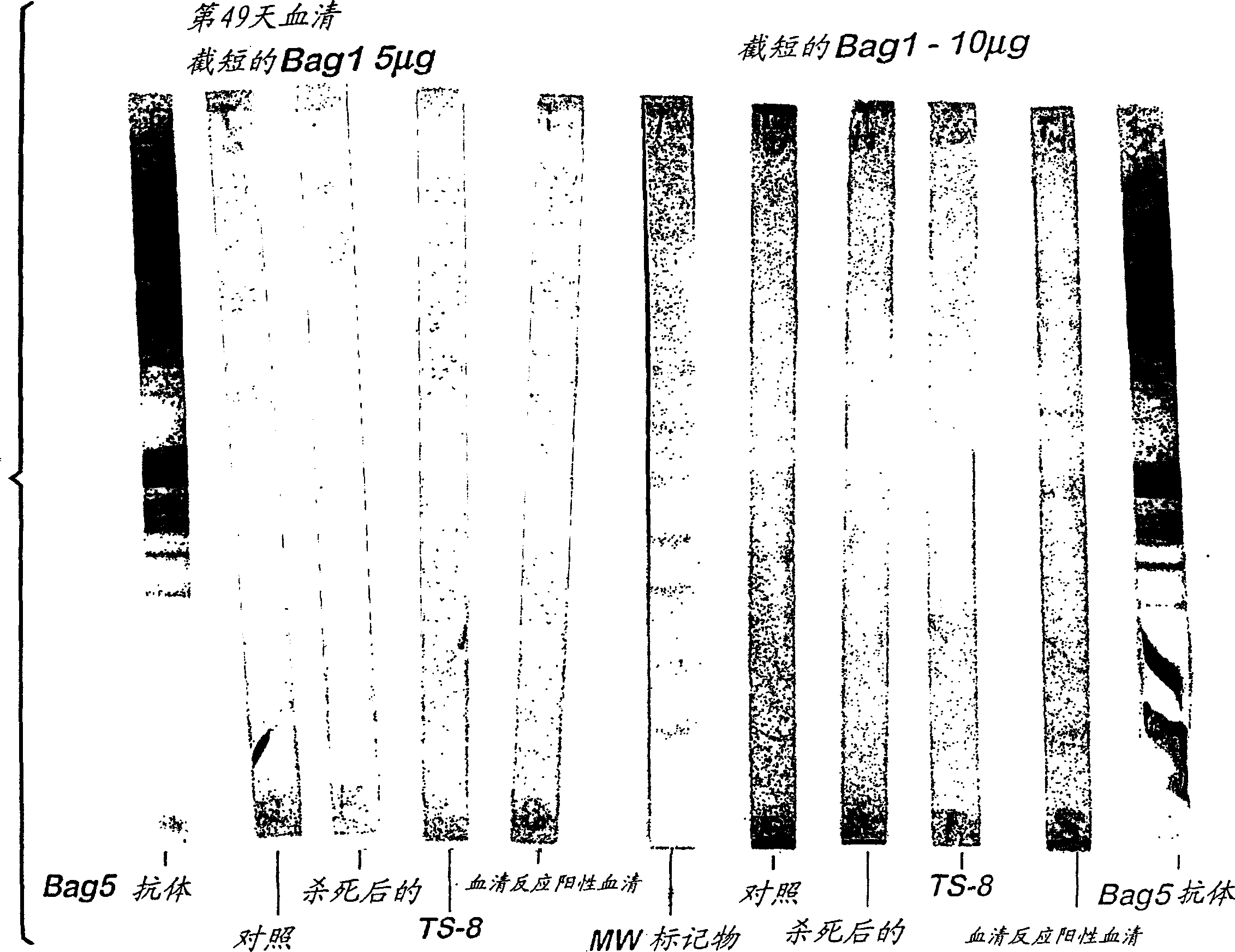
The invention relates to a modification method and an application of a neospora caninum surface protein SRS2 gene and belongs to the technical field of genetic engineering. On the basis of complete SRS2 genes, 168 basic groups are truncated from an N-terminal, 75 basic groups are truncated from a C-terminal, the truncated tSRS2 gene has the sequence of SEQ ID No.1, a target gene E.Coli codon optimization and inducible expression and purification of target protein are completed, and a kit is used for clinically detecting neosporiasis.
Owner:CHONGQING AULEON BIOLOGICALS
Polynucleotide molecules encoding neospora proteins
The present invention provides isolated polynucleotide molecules comprising nucleotide sequences encoding GRA1, GRA2, SAG1, MIC1 and MAG1 proteins from Neospora caninum, as well as recombinant vectors, transformed host cells, and recombinantly-expressed proteins. The present invention further provides a polynucleotide molecule comprising the nucleotide sequence of the bidirectional GRA1IMAGI promoter of N. caninum. The present invention further provides genetic constructs based on the polynucleotide molecules of the present invention that are useful in preparing modified strains of Neospora cells for use in vaccines against neosporosis.
Owner:PFIZER INC
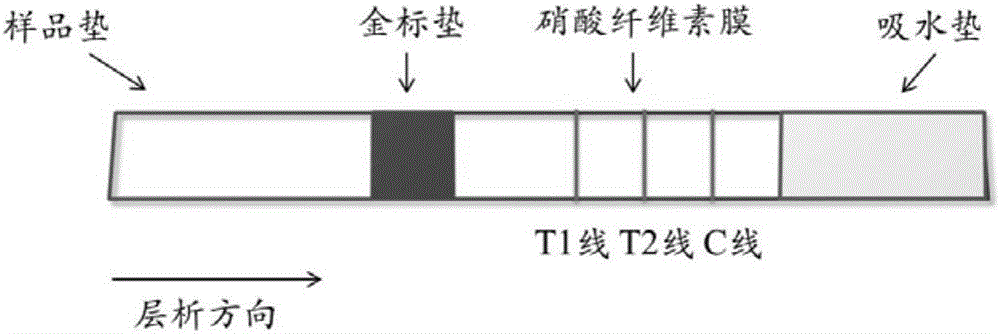
Bigeminal colloidal gold test strip for detecting toxoplasma gondii and neospora caninum antibodies
InactiveCN105785031AStrong characteristicIncreased sensitivityBiological testingImmunoassaysSerum igeNeospora species
The invention discloses a bigeminal colloidal gold test strip for detecting toxoplasma gondii and neospora caninum antibodies, and provides a bigeminal colloidal gold test strip for detecting toxoplasma gondii and / or neospora caninum. The bigeminal colloidal gold test strip comprises a bottom plate, as well as a sample pad, a colloidal gold pad, a nitrocellulose membrane and an absorbent pad, which are arranged on the bottom plate, wherein the colloidal gold pad is coated with a colloidal gold protein; the nitrocellulose membrane contains a detection line T1, a detection line T2 and a quality control line C. Experiments show that the prepared bigeminal colloidal gold test strip can be used for simultaneously detecting plasma antibodies of toxoplasma gondii and neospora caninum in an animal body and is convenient to operate and applicable to detection of animals such as pigs, dogs and cats, differential diagnosis of infections caused by the two animal protozoans is facilitated and the cost is reduced.
Owner:CHINA AGRI UNIV
Parasitic protozoan isolate
InactiveUS20030185852A1Avoid abortionReduce the possibilityBiocideProtozoaNeospora speciesTeliospore
Owner:UNIV OF TECH SYDNEY
Bovine pathogen array
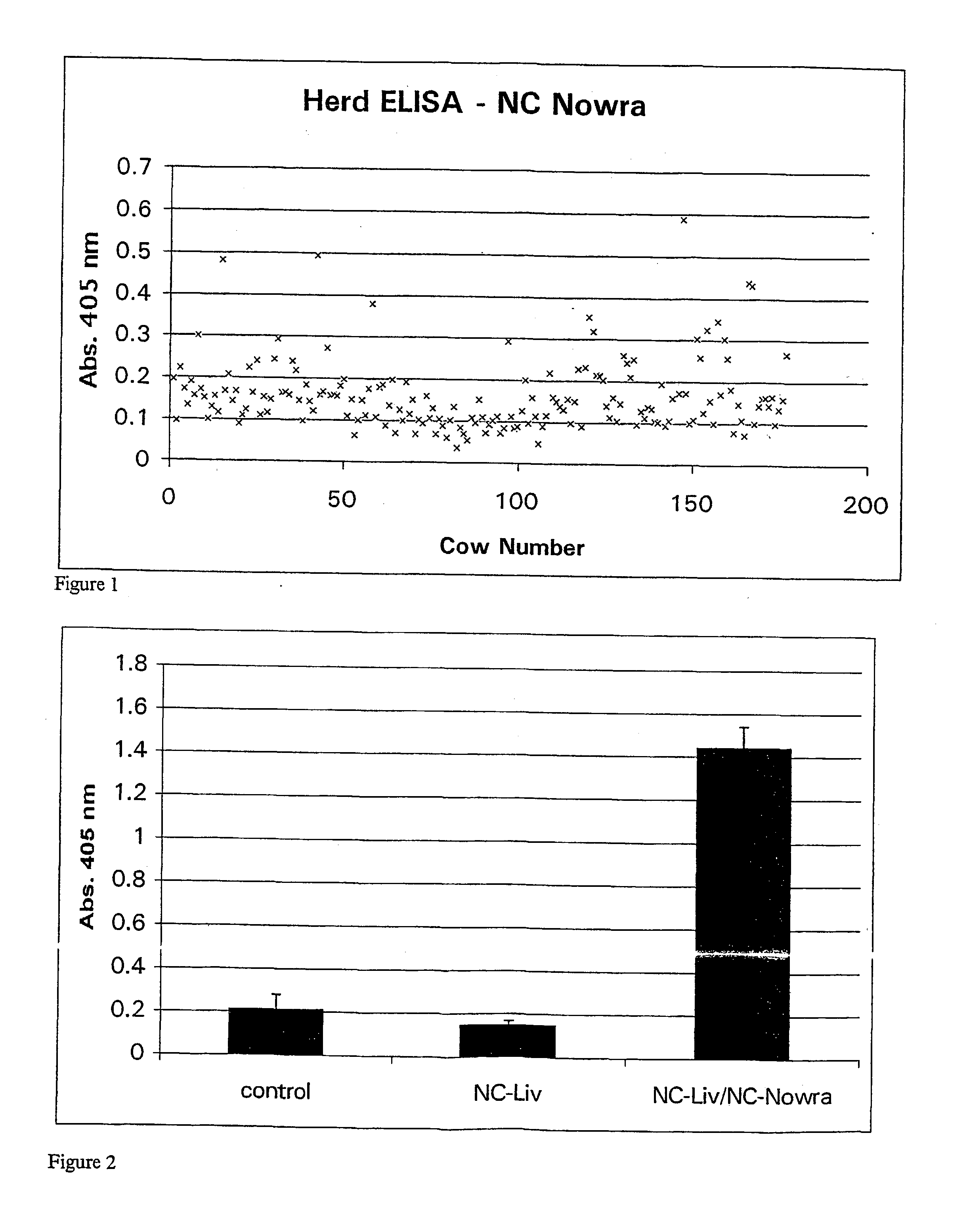
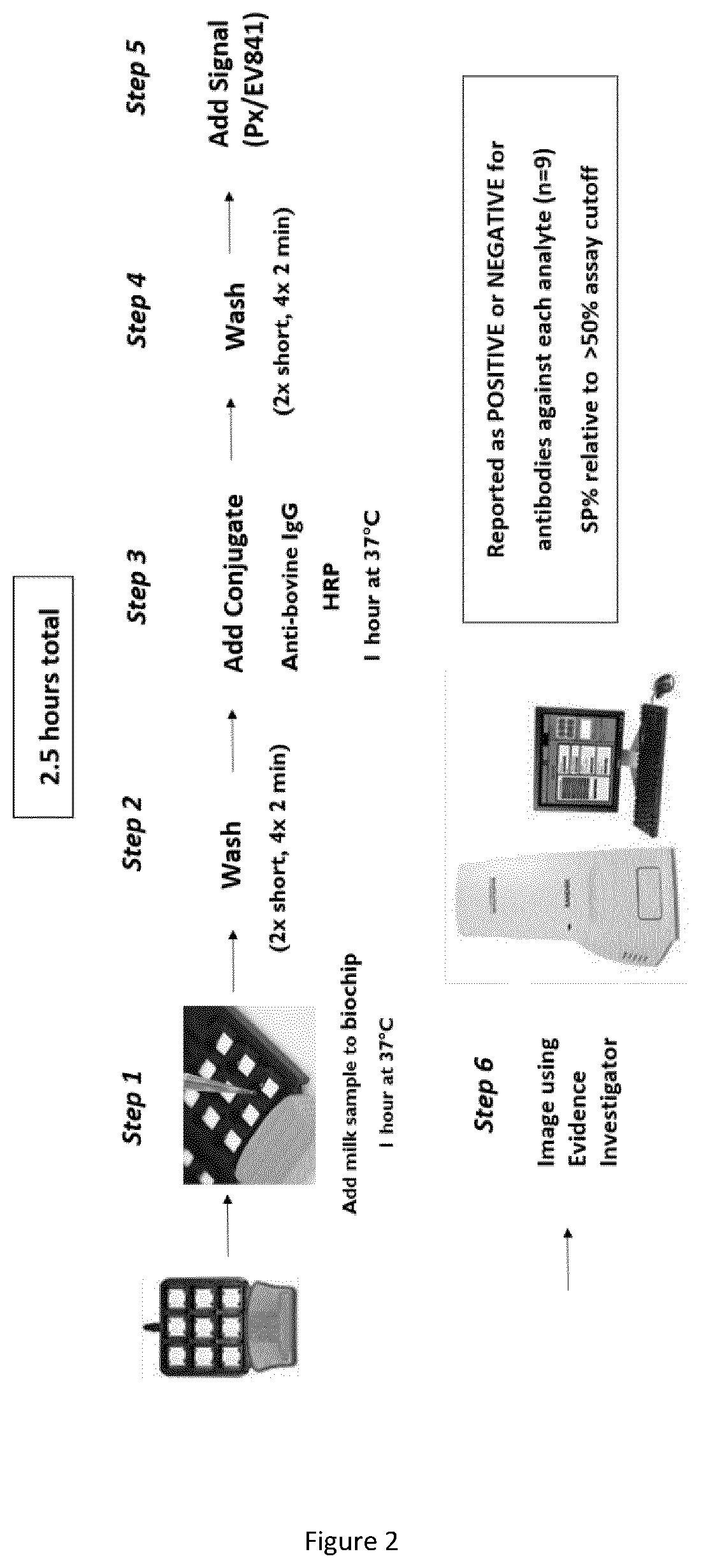
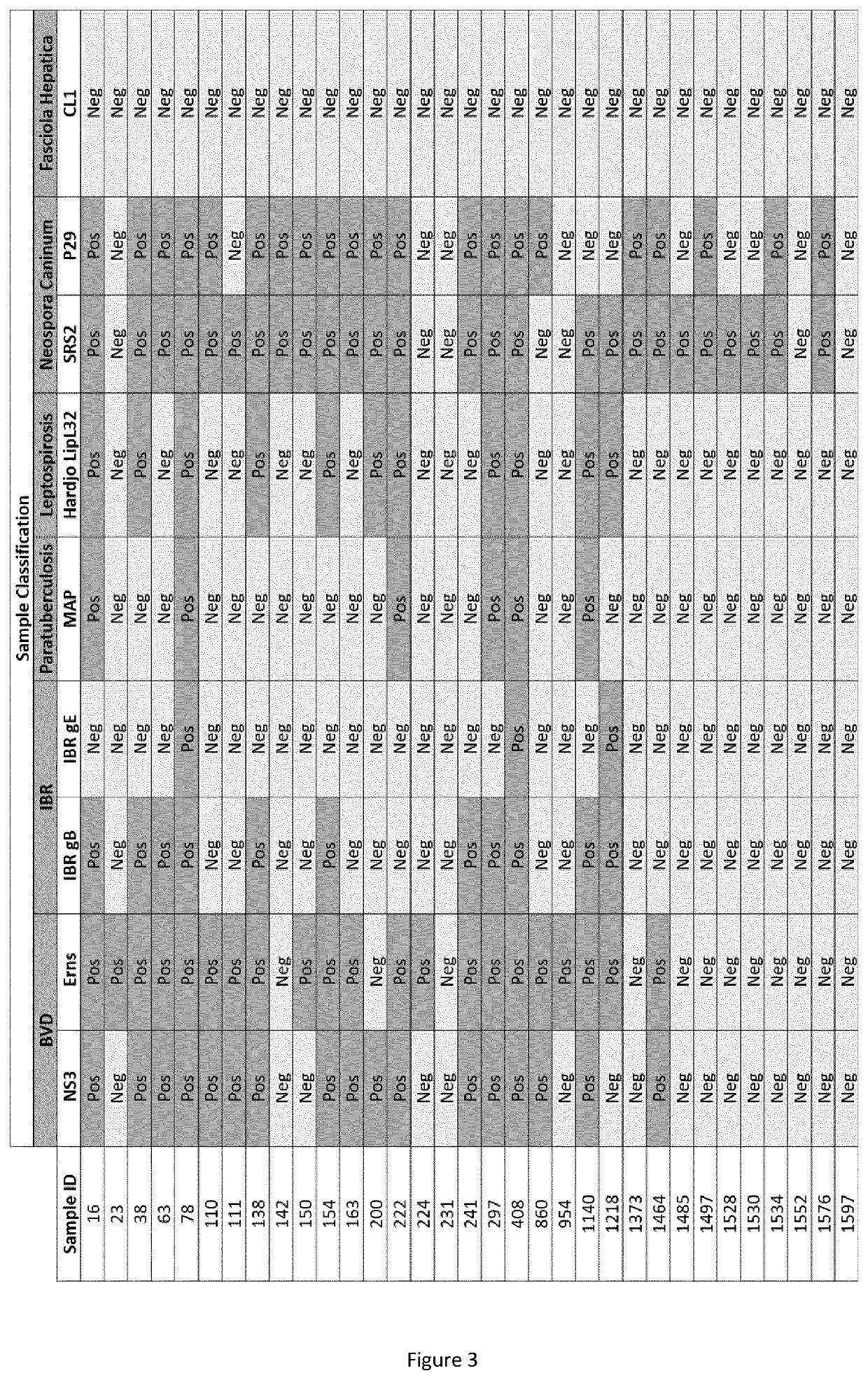
The current invention provides a multiplex method for screening bovine samples for antibodies against several important pathogens. These pathogens include Bovine Viral Diarrhoea Virus (BVDV), Bovine Herpesvirus-1 (BoHV-1), Mycobacterium paratuberculosis (MAP), Leptospira species, Neospora caninum and Fasciola hepatica. This multiplex screening is enabled by substrates with immobilized pathogen antigens and offer multiple advantages for the routine testing of farm animals.
Owner:RANDOX LAB LTD +1
Serological assay for Neospora caninum
InactiveUS20050112567A1Microbiological testing/measurementBiological testingNeospora speciesWestern blot
The present invention relates to the serological detection of Neospora caninum-vaccinated animals by means of reaction of a subject serum with a protein reactive with N. caninum. The protein may be a full-length native or recombinant N. caninum or Toxoplasma gondii bradyzoite fusion protein or a truncated fusion protein or fragment thereof. The protein may be used in any of a number of assays including enzyme linked immunosorbant assays (ELISA), a radioimmunoassay (RIA), a Western Blot and other suitable forms of immunoassay.
Owner:PFIZER INC +1
Vaccine preparation for neospora caninum infection
InactiveUS8821882B2High prophylactic effectReduce riskPeptide/protein ingredientsSnake antigen ingredientsAntigenSpore
A vaccine preparation characterized in that Neospora caninum-derived dense granule protein 7 or apical membrane antigen 1 or an immunologically active variant or derivative thereof is included in liposomes each having an oligosaccharide capable of binding to a carbohydrate recognition molecule on the surface of antigen-presenting cells on the surface of the liposome.
Owner:OBIHIRO UNIVERSITY OF AGRICULTURE AND VETERINARY MEDICINE +1
Vaccine for prevention and control of neosporiasis of cattle as well as preparation method and application of vaccine
InactiveCN104784701AImprove immunityImprove transgenic efficiencyGenetic material ingredientsPharmaceutical non-active ingredientsBuprestis novemmaculataOpen reading frame
The invention provides a vaccine for prevention and control of neosporiasis of cattle as well as a preparation method and an application of the vaccine. The vaccine is a gene adjuvant recombinant adenovirus carrier vaccine, and the nucleotide sequence of a vaccine antigen is encoded as in SEQ.ID.NO.1. A preparation method of the vaccine comprises steps as follows: primers are designed according to open reading frames of an NcSRS 9 gene sequence and an IFN-gamma gene sequence of Neospora caninum, an NcSRS 9 gene and an IFN-gamma gene are amplified and spliced, a pMD18T-NcSRS9-IFN-gamma recombinant clone plasmid is built, a shuttle plasmid pCR259-NcSRS9-IFN-gamma and an expression plasmid H294-NcSRS9-IFN-gamma of a recombinant adenovirus are built, Ad5-NcSRS9-IFN-gamma recombinant adenoviruses are packed with QBI-HEK293 cells through lipidosome mediation and transfection, and amplification and purification are performed to prepare the recombinant adenovirus carrier vaccine.
Owner:YANBIAN UNIV
Neospora caninum specific PCR detection kit and preparation method and application
ActiveCN111197100BStrong specificityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationSporeNeospora species
The invention discloses a Neospora caninum specific PCR detection kit and its preparation method and application, wherein the specific gene sequence of Neospora caninum is SEQ ID NO.1, and the specific gene sequence has five further designed the amplification primers of the specific gene sequence and constructed the Neospora caninum PCR detection kit, which is intended to be applied to the clinical detection and screening of Neospora caninum infection in animals, and can accurately and rapidly diagnose Neospora caninum infection. Caused by the pathogen of female abortion and the investigation of Neospora infection in animal populations.
Owner:CHINA AGRI UNIV
The medical application of compound pyrogallol in anti-Neospora canis
InactiveCN109908117BAvoid infectionHydroxy compound active ingredientsAntiparasitic agentsNeospora speciesChemical compound
Owner:JILIN UNIV
Neosporosis recombinant enzyme-linked immunosorbent assay (rELISA) antibody assay kit
InactiveCN102455360BHybrid peptidesVector-based foreign material introductionEscherichia coliAntigen
The invention provides a neosporosis rELISA antibody assay kit. The neosporosis recombinant enzyme-linked immunosorbent assay (rELISA) antibody assay kit is characterized in that designing a primer set according to a neospora caninum NcSRS2 gene of the GenBank, introducing the primer set to an enzyme digestion site, removing a signal peptide zone of an NcSRS2 protein, extracting a DNA from Xinjiang cow positive serum, carrying out polymerase chain reaction (PCR) amplification of the DNA to obtain a tNcSRS2 genetic fragment having length of 975bp, cloning the tNcSRS2 genetic fragment into a pMD-18-T carrier, carrying out enzyme digestion and sequencing, further directionally connecting the desired fragment obtained by the previous step to a pGEX-4T-2 expression carrier, transferring the pGEX-4T-2 expression carrier with the desired fragment into escherichia coli, carrying out inducible expression and purification, wherein expressed fusion protein molecular weight is about 62kDa, and carrying out ELISA antigen coating by the expressed fusion protein to obtain the neosporosis rELISA antibody assay kit. A use method of the neosporosis rELISA antibody assay kit comprises the following steps that a coated enzyme label reaction plate is taken out, is subjected to first washing, is enclosed, is subjected to second washing, is added with a sample needing to be detected, is subjected to third washing, is added with a secondary antibody, is subjected to fourth washing, and undergoes a color reaction; and when the color reaction is finished, an OD450 / 630 value of the coated enzyme label reaction plate is read and a result is determined. Compared with a commercial ELISA assay kit, the neosporosis rELISA antibody assay kit has a higher coincidence rate above 95% The neosporosis rELISA antibody assay kit also is suitable for the ELISA of a neospora caninum antibody in ruminant serum and blood plasma.
Owner:XINJIANG AGRI UNIV
A sarcocystis fusion antigen, coding gene, indirect ELISA antibody detection kit and application thereof
ActiveCN110922491BGood antigenicityStrong characteristicBacteriaAntibody mimetics/scaffoldsSarcocystis cruziNeospora species
The invention discloses a sarcocystis fusion antigen, coding gene, indirect ELISA antibody detection kit and application thereof, belonging to the technical field of animal or human disease detection. The sarcocystis fusion antigen of the present invention consists of the partial amino acid sequence (37-118) of the surface antigen SAG3 of Sarcocystis mieni, a flexible peptide of 15 amino acids and the partial amino acid sequence of the surface antigen SAG4 of Sarcocystis cruzi (No. 165‑264) constitutes; this fusion antigen has very strong antigenicity and specificity, can produce effective antigen antibody reaction with S. The positive sera of Toxoplasma gondii and Neospora caninum produce cross-immune reactions, which are ideal detection antigens for detecting antibodies against Sarcocystis micheni and Sarcospora cruzi, and have a good application prospect.
Owner:HENAN UNIV OF SCI & TECH
Sarcocystis fusion antigen, coding gene, indirect ELISA antibody detection kit and application thereof
ActiveCN110922491AGood antigenicityStrong characteristicBacteriaAntibody mimetics/scaffoldsSarcocystis cruziNeospora species
The invention discloses a sarcocystis fusion antigen, a coding gene, an indirect ELISA antibody detection kit and application thereof, and belongs to the technical field of animal or human epidemic disease detection. The sarcocystis fusion antigen consists of a part of amino acid sequences (37 th-118 th sites) of a surface antigen SAG3 of sarcocystis miescheriana, 15 amino acid flexible peptides and a part of amino acid sequences (165 th-264 th sites) of a surface antigen SAG4 of sarcocystis cruzi. The fusion antigen has very high antigenicity and specificity, can generate effective antigen-antibody reaction with sarcocystis miescheriana and sarcocystis cruzi antibodies as a detection antigen, cannot generate cross immune reaction with positive serum of toxoplasma gondii and neospora caninum, is an ideal detection antigen for detecting the sarcocystis miescheriana and sarcocystis cruzi antibodies, and has good application prospects.
Owner:HENAN UNIV OF SCI & TECH
Vaccine for the prevention and control of bovine neosporosis and its preparation method and application
InactiveCN104784701BImprove immunityImprove transgenic efficiencyGenetic material ingredientsPharmaceutical non-active ingredientsBuprestis novemmaculataOpen reading frame
The invention provides a vaccine for prevention and control of neosporiasis of cattle as well as a preparation method and an application of the vaccine. The vaccine is a gene adjuvant recombinant adenovirus carrier vaccine, and the nucleotide sequence of a vaccine antigen is encoded as in SEQ.ID.NO.1. A preparation method of the vaccine comprises steps as follows: primers are designed according to open reading frames of an NcSRS 9 gene sequence and an IFN-gamma gene sequence of Neospora caninum, an NcSRS 9 gene and an IFN-gamma gene are amplified and spliced, a pMD18T-NcSRS9-IFN-gamma recombinant clone plasmid is built, a shuttle plasmid pCR259-NcSRS9-IFN-gamma and an expression plasmid H294-NcSRS9-IFN-gamma of a recombinant adenovirus are built, Ad5-NcSRS9-IFN-gamma recombinant adenoviruses are packed with QBI-HEK293 cells through lipidosome mediation and transfection, and amplification and purification are performed to prepare the recombinant adenovirus carrier vaccine.
Owner:YANBIAN UNIV
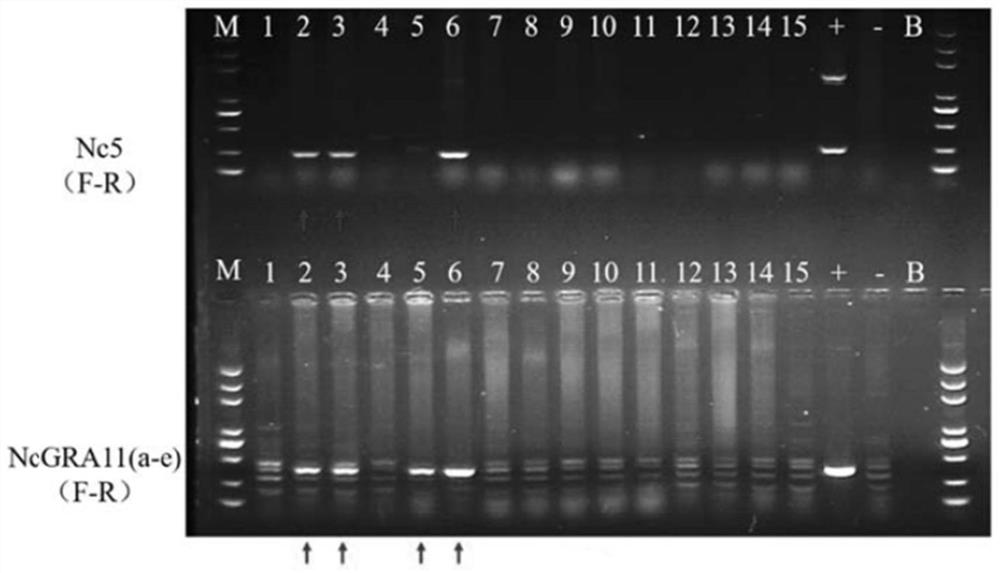
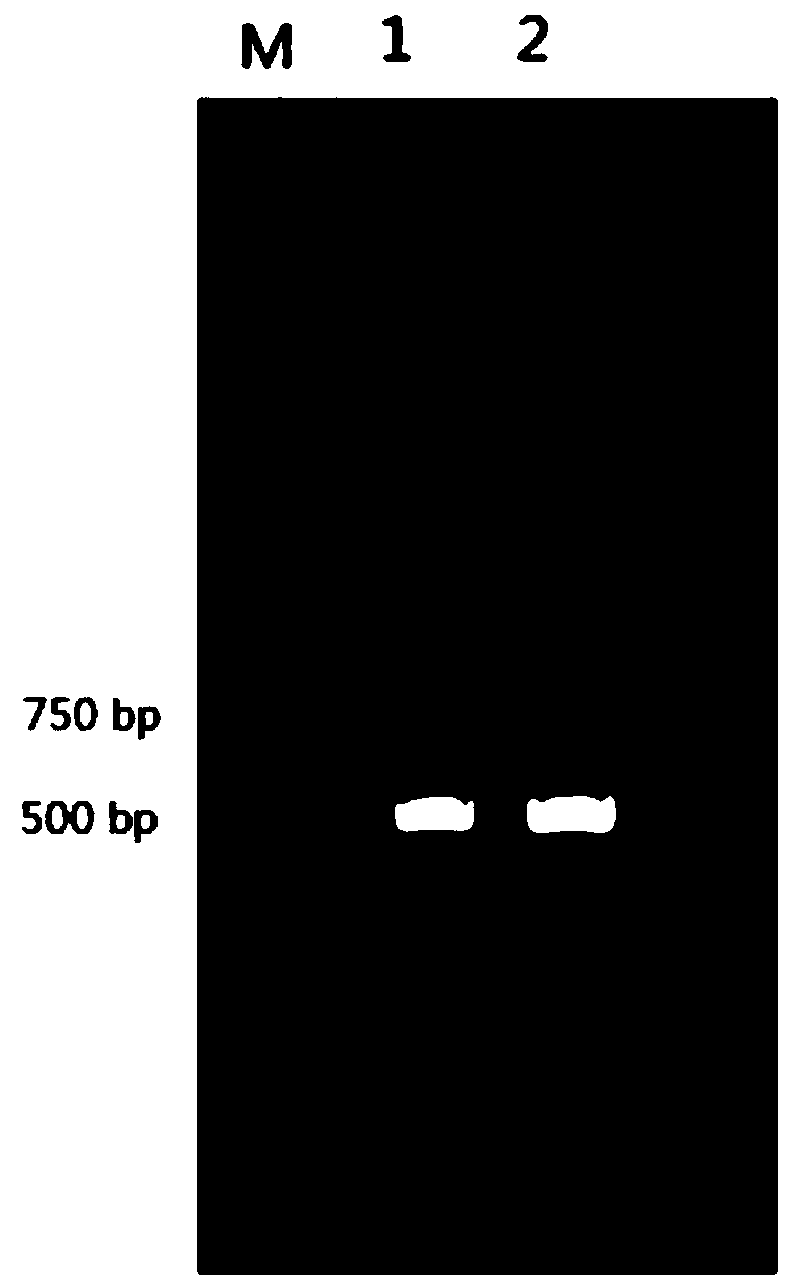
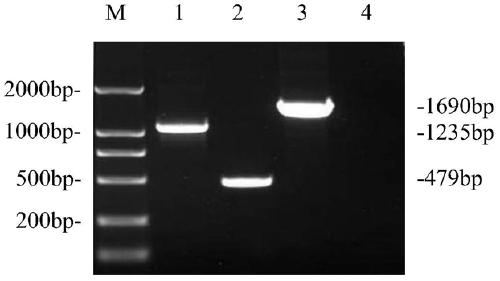
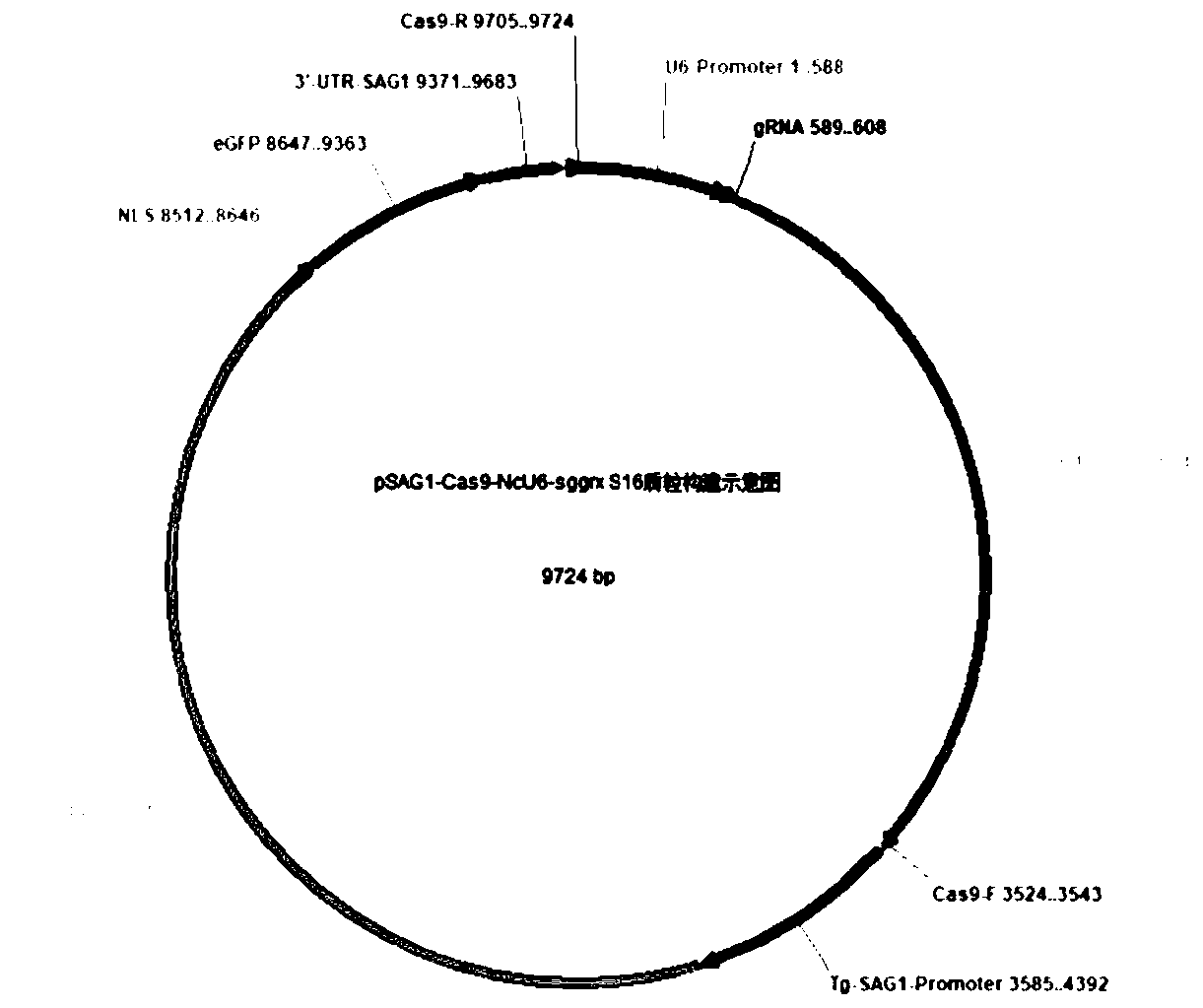
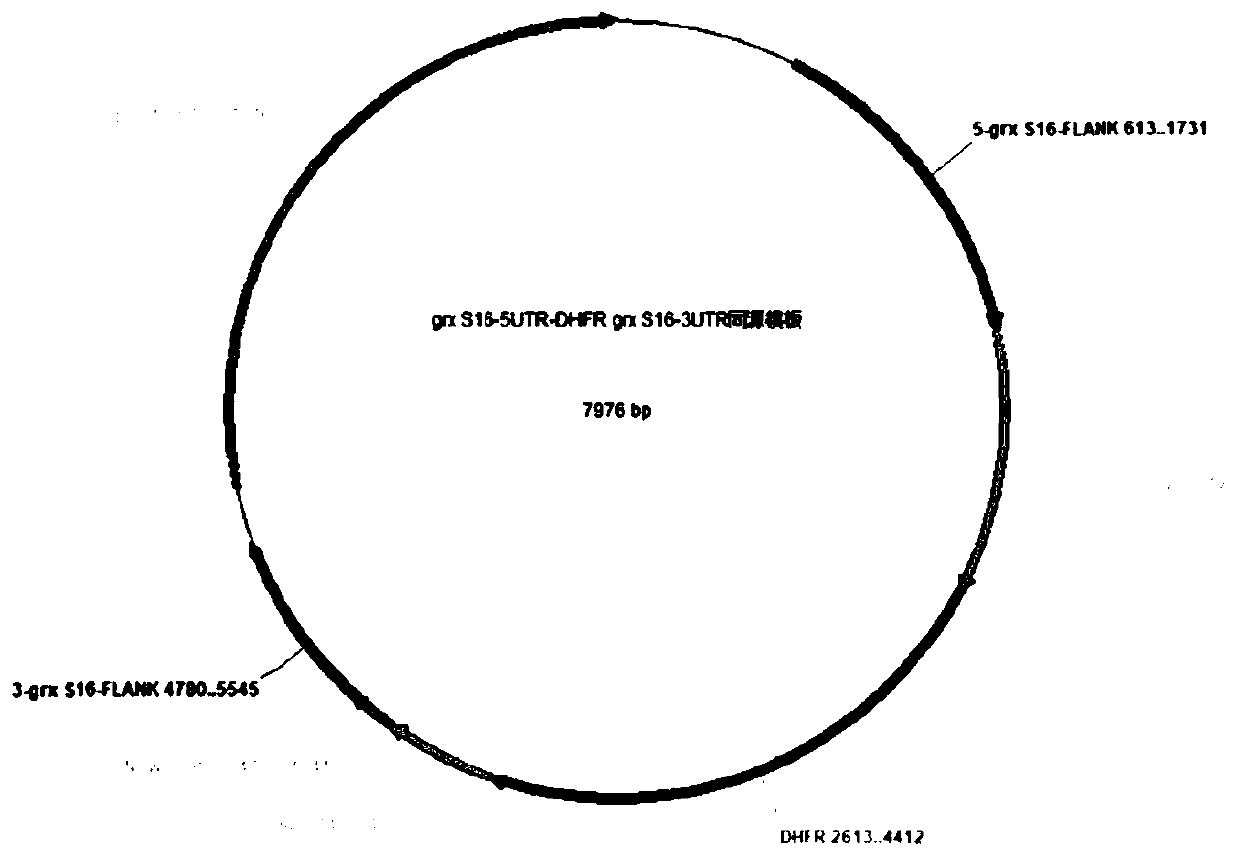
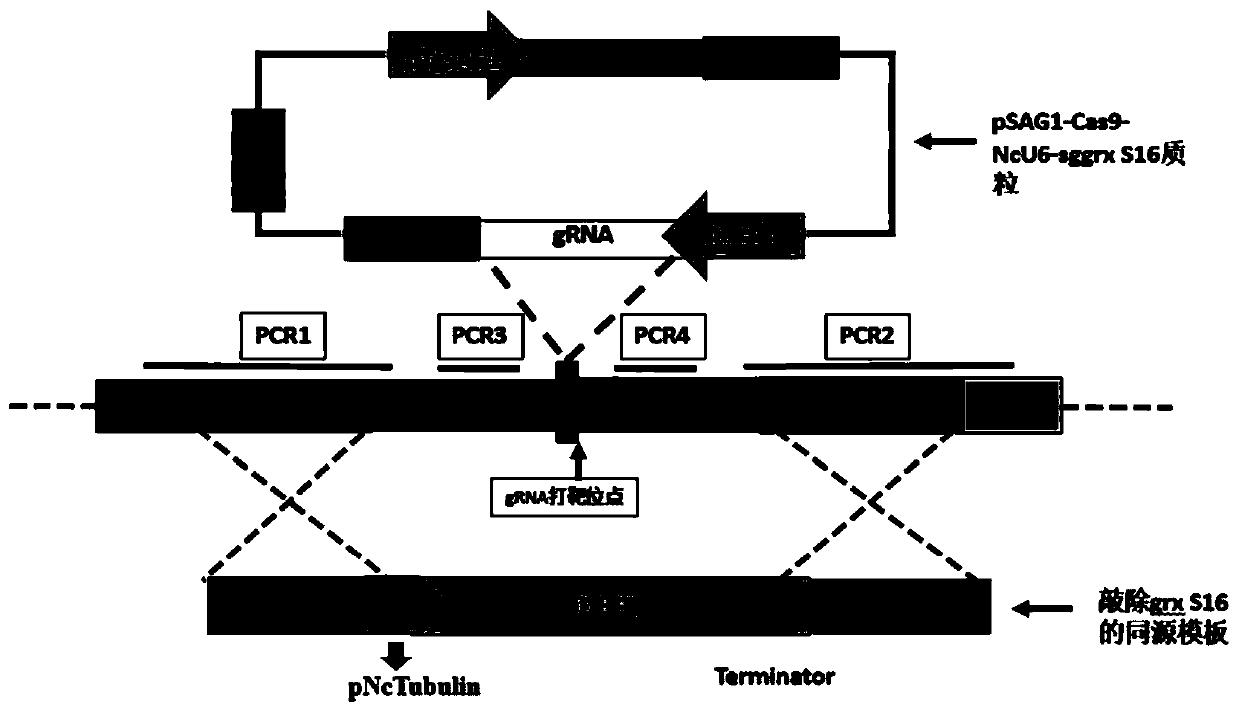
Neospora caninum attenuated strain with double deletion of grx S16 and grx C5 genes, and construction method and application thereof
ActiveCN111187722AAvoid reinfectionReduce pathogenicityProtozoa antigen ingredientsProtozoaNeospora speciesNucleotide
The invention discloses a neospora caninum attenuated strain with the double deletion of grx S16 and grx C5 genes, and a construction method and application thereof. The strain lacks the glutaredoxinS16 gene (grx S16) and glutaredoxin C5 gene (grx C5) of neospora caninum, and nucleotide sequences of the grx S16 gene and grx C5 gene are as shown in SEQ ID NO.1 and SEQ ID NO.2, respectively. The strain with the double deletion of grx S16 and grx C5 genes of neospora caninum constructed by the invention is significantly reduced in reproductive capacity and virulence, can effectively resist the reinfection of neospora caninum after inoculation of mice, and has the potential to become a neospora caninum attenuated vaccine.
Owner:CHINA AGRI UNIV
Neospora caninum specific PCR detection kit, and preparation method and application thereof
ActiveCN111197100AStrong specificityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationNeospora speciesGenome
The invention discloses a Neospora caninum specific PCR detection kit, and a preparation method and an application thereof. The specific gene sequence of the Neospora caninum is represented by SEQ IDNO.1. The specific gene sequence has five copies in a Neospora caninum genome. An amplification primer of the specific gene sequence is further designed, and the Neospora caninum PCR detection kit isconstructed, and is applied to clinical detection and screening of animal Neospora caninum infection to accurately and quickly diagnose female animal abortion pathogens caused by Neospora caninum infection and investigate animal population Neospora caninum infection.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com