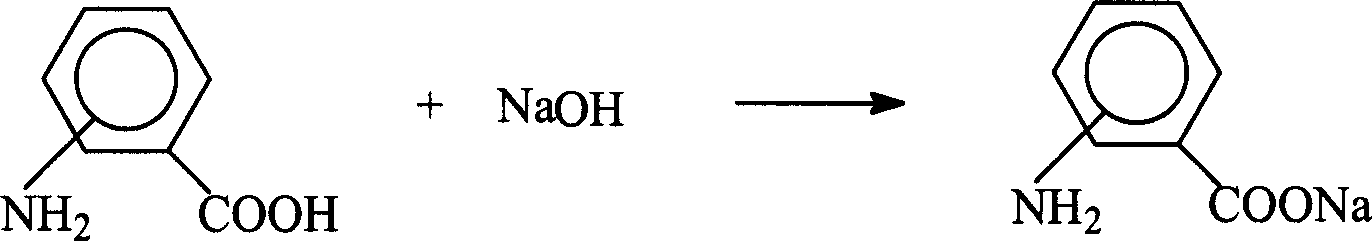Positive-negative ion type bimetal catalyst, and its preparing method and use
A bimetallic catalyst, positive and negative ion technology, applied to the preparation of carboxylic acid by carbon monoxide reaction, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as deactivation, achieve easy generation, improve catalytic activity and stability, The effect of promoting the effect of catalysis is improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0032] Example 1
[0033] Preparation of catalyst anion part non-rhodium metal complex:
[0034] Weigh 0.02 mol of aminobenzoic acid and NaOH in 2 mol of water and heat to 70°C to react for 1 hour. After cooling, precipitate with excess acetone. After drying, sodium aminobenzoate is obtained. The reaction and structural formula are as follows:
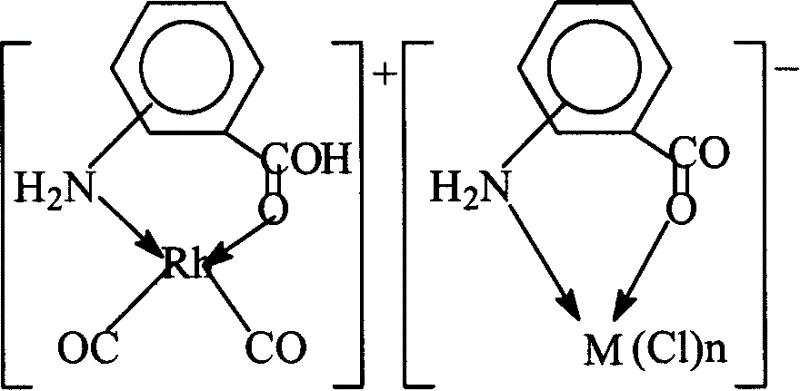
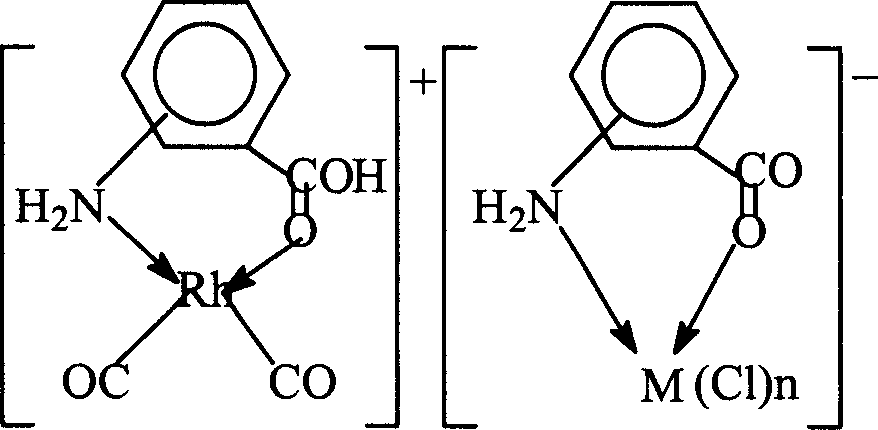
[0035]
[0036] Weigh 0.01 mol of sodium aminobenzoate and dissolve it in a mixture of 1 mol of methanol and 0.5 mol of water, and add 0.01 mol of RuCl 3 , The reaction was heated and refluxed under stirring for 1 hour, after cooling, precipitated with acetone, and dried to obtain the sodium aminobenzoate ruthenium complex, the reaction and complex structure are as follows:
[0037]
[0038] The chromium, tin, lead and zirconium complexes of aminobenzoic acid sodium salt can also be obtained according to the above method. In this example, for the sake of brevity, they are not listed one by one.
[0039] Preparation of positive and nega...
Example Embodiment
[0045] Example 2
[0046] In a 250ml zirconium autoclave was added 0.32g of the rhodium-ruthenium bimetallic catalyst prepared in Example 1 with anthranilic acid as ligand, 1.24mol of methanol, 0.87mol of acetic acid, and 0.24mol of methyl iodide, and the temperature was raised after the introduction of CO To 150°C, keep the reaction pressure 4.0 MPa, the stirring speed 500 rpm, and the reaction time 30 min. The methanol conversion rate is 100%, the methyl acetate content is 0.02 mol, the acetic acid increment is 1.20 mol, and the acetic acid space-time yield is 20.7 mol AcOH / L·h.
Example Embodiment
[0047] Example 3
[0048]Add 0.32g of rhodium-chromium bimetallic catalyst with anthranilic acid as ligand prepared according to the method of Example 1 into the autoclave, 1.24mol of methanol, 0.87mol of acetic acid, and 0.24mol of methyl iodide. After passing CO, the temperature is raised to 180°C, keep reaction pressure 4.0MPa, stirring speed 500rpm, reaction time 20min, methanol conversion rate 100%, methyl acetate content is 0.04mol, acetic acid increment is 1.1mol, acetic acid space-time yield is 31.34mol AcOH / L·h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com