Preparation method of iron-nickel bimetallic selenide nanosphere electrocatalyst
A catalyst and bimetallic technology, applied in selenium/tellurium compounds, chemical instruments and methods, elemental compounds other than selenium/tellurium, etc., can solve problems such as poor conductivity, achieve improved electrochemical stability, and simple preparation process The effect of convenience and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 8 g of flaky sodium hydroxide in 120 mL of deionized water and stir until the solution is clear.
[0036] Mix 0.5 g of Se powder and 20 mL of N 2 H 4 ·H 2 O was added to the above solution and stirred for 0.5h.
[0037] Then add 0.75g of Ni (NO 3 ) 2 ·6H 2 O and 0.26g Fe(NO 3 ) 3 ·9H 2 O powder, stir again for 3h.
[0038] Centrifuge and wash with deionized water and anhydrous ethanol for 3 times respectively, and then put it into a drying oven at 70 °C for 16 h. The obtained product is an efficient iron-nickel bimetallic selenide nanosphere electrocatalyst.
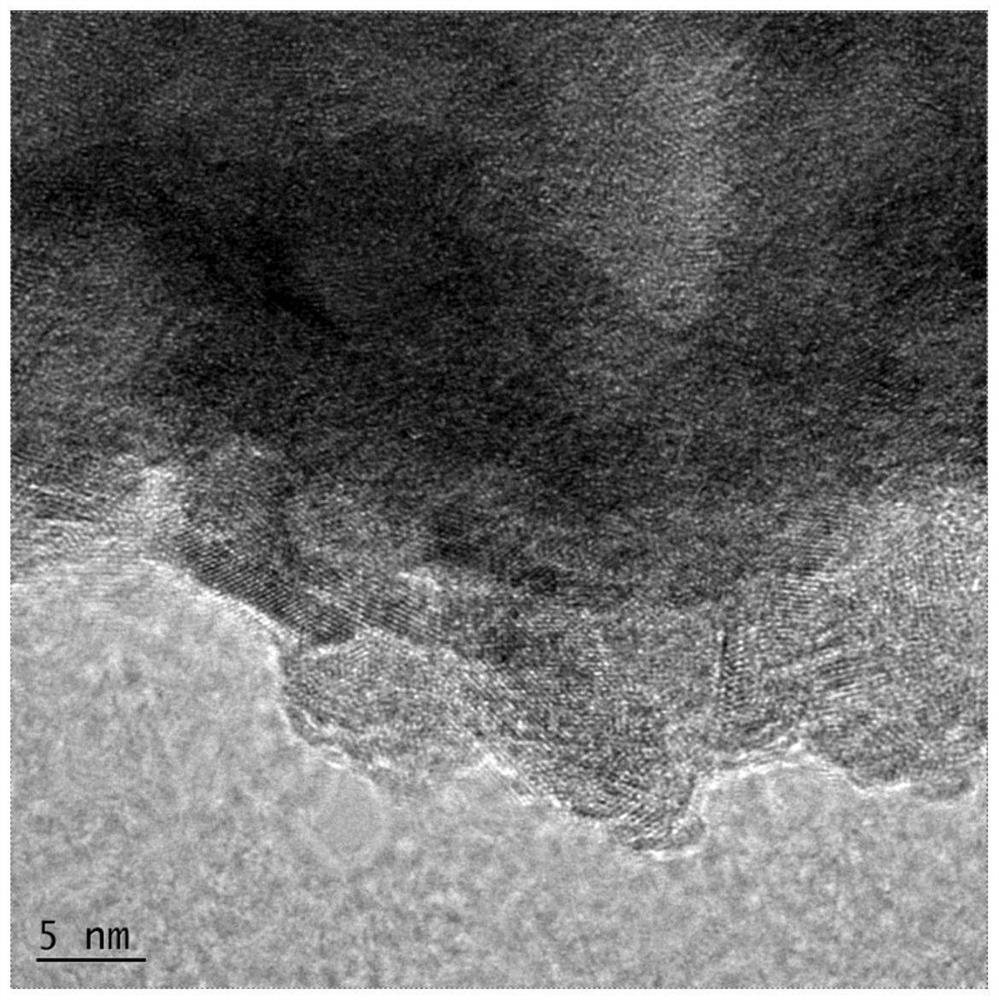
[0039] SEM images and HRTEM images of the prepared nanosphere electrocatalysts were detected, and the results were as follows figure 1 , figure 2shown. It is found that the nanosphere electrocatalyst is composed of a porous framework composed of nano-microspheres with uniform distribution of elements, which increases the active site and electron transport rate, and can clearly see Fe 0.17 Ni...
Embodiment 2
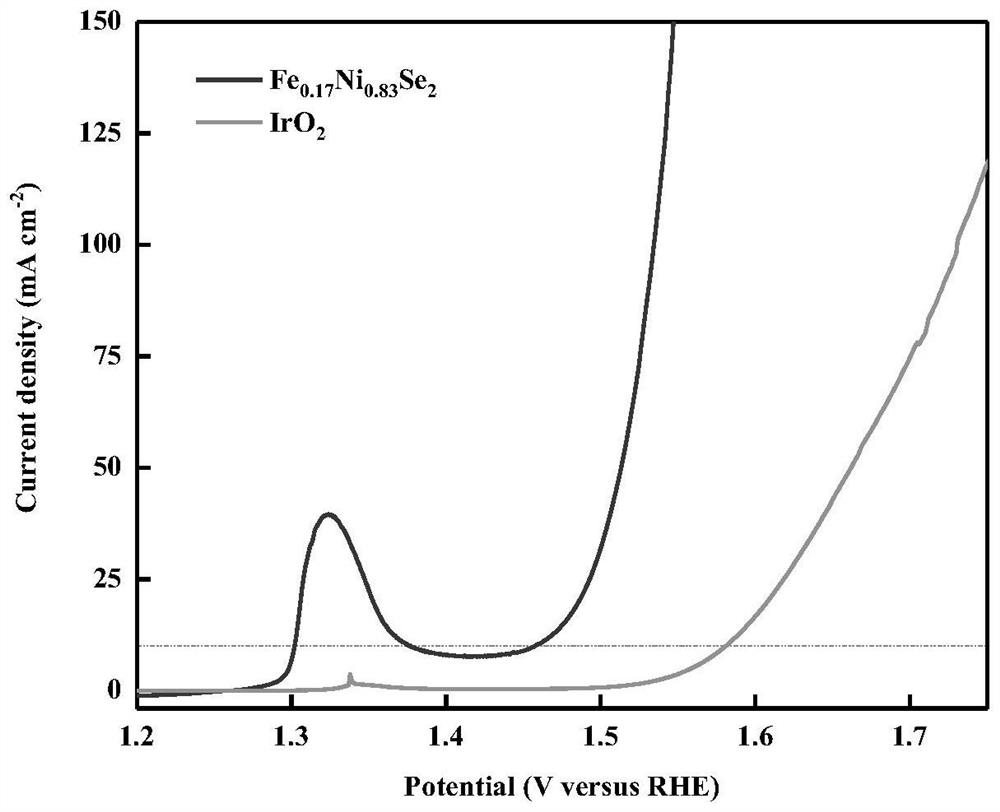
[0041] The inks were made and tested for electrochemical performance. Take 5mg of the prepared iron-nickel bimetallic selenide nanosphere electrocatalyst, add 25ul of Nafion, 200ul of deionized water and 800ul of deionized ethanol, ultrasonically treat for 30min, take 250ul of ink and drop it on a foam with an area of 1×1.5cm On nickel, and vacuum-dried at 60 °C for 4 h, all tests were carried out using Chenhua CHI760E electrochemical workstation, the test temperature was room temperature, the electrolyte was 1M KOH solution, and the iR compensation was 95%.
[0042] Take commercial IrO 2 Electrocatalyst 5mg, add 25ul Nafion, 200ul deionized water and 800ul absolute ethanol, ultrasonically treat for 30min, take 250ul ink drop on nickel foam with an area of 1 × 1.5cm, and vacuum dry at 60 ° C for 4h, All tests were performed using Chenhua CHI760E electrochemical workstation, the test temperature was room temperature, the electrolyte was 1MKOH solution, and the iR compensat...
Embodiment 3
[0046] In addition to Se powder, Ni (NO 3 ) 2 ·6H 2 O and Fe (NO 3 ) 3 ·9H 2 Except for the different amount of O added, other experimental procedures are the same as those in Example 1. Se powder, Ni (NO 3 ) 2 ·6H 2 O and Fe (NO 3 ) 3 ·9H 2 The addition amount of O and the performance data of the obtained nanosphere electrocatalyst are shown in Table 1.
[0047] Table 1
[0048]
[0049] Through Example 1, Example 3 and Table 1, it can be found that the products prepared by Fe, Ni and Se with different atomic ratios also have certain differences in overpotential. Although the overpotential of the product can be reduced to less than 300mV when the atomic ratio of Ni is 0, when the atomic ratio of Fe and Ni is 17:83, the overpotential of the product can be reduced to 227mV. Under this ratio, Fe and Ni synergize. Unexpected technical results have been achieved.
[0050] The invention provides a preparation method and application of an efficient iron-nickel bimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com