Electrocatalytic ammonia oxidation hydrogen production electrode material as well as preparation method and application thereof
An electrode material and ammoxidation technology, applied in the field of electrocatalytic materials, can solve the problems of limiting the current density and stability of Pt-based catalysts, difficulty in realizing large-scale production and application, and lack of precious metal Pt resources, so as to improve the catalytic stability of ammoxidation , low raw material cost, catalytic activity and stability improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
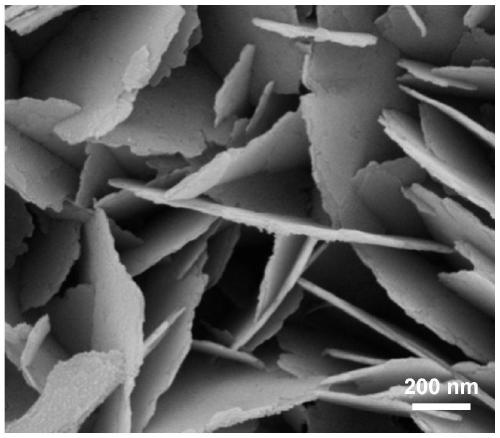
[0031] (1) Pretreatment of foamed nickel: Cut commercially available nickel foam into a size of 3cm×5cm, immerse in acetone, and ultrasonicate for 15min. Remove oil stains on the surface, wash with water 3 times, each time for 5 minutes, and wash off the acetone; immerse in 3mol / L hydrochloric acid, ultrasonicate for 5 minutes, remove the oxide film on the surface of foamed nickel, then wash with water 3 times, each time for 3 minutes, remove Cl - , submerged in ethanol, ultrasonicated for 5 minutes, washed with water three times, each time for 3 minutes, and dried under vacuum conditions for later use.
[0032] (2) Dissolve 2mmol of ammonium fluoride and 5mmol of urea in 50mL of deionized water, and after magnetically stirring for 15min, transfer the mixed solution into a 100mL reaction kettle, and then add the cleaned 3cm×5cm nickel foam into it; Place it in a constant temperature box at 120°C, react for 12 hours; cool down naturally, wash, and dry in vacuum at 60°C to obtai...
Embodiment 2
[0035] (1) Commercially available nickel foam is pretreated according to Example 1.
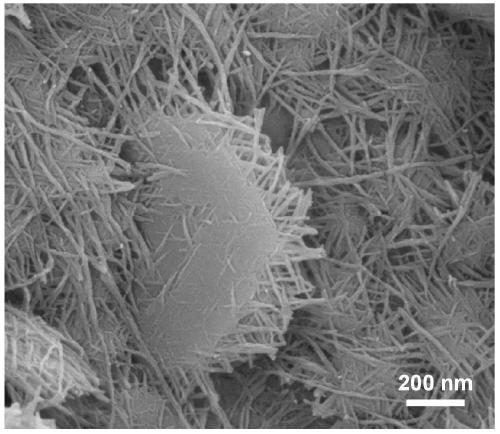
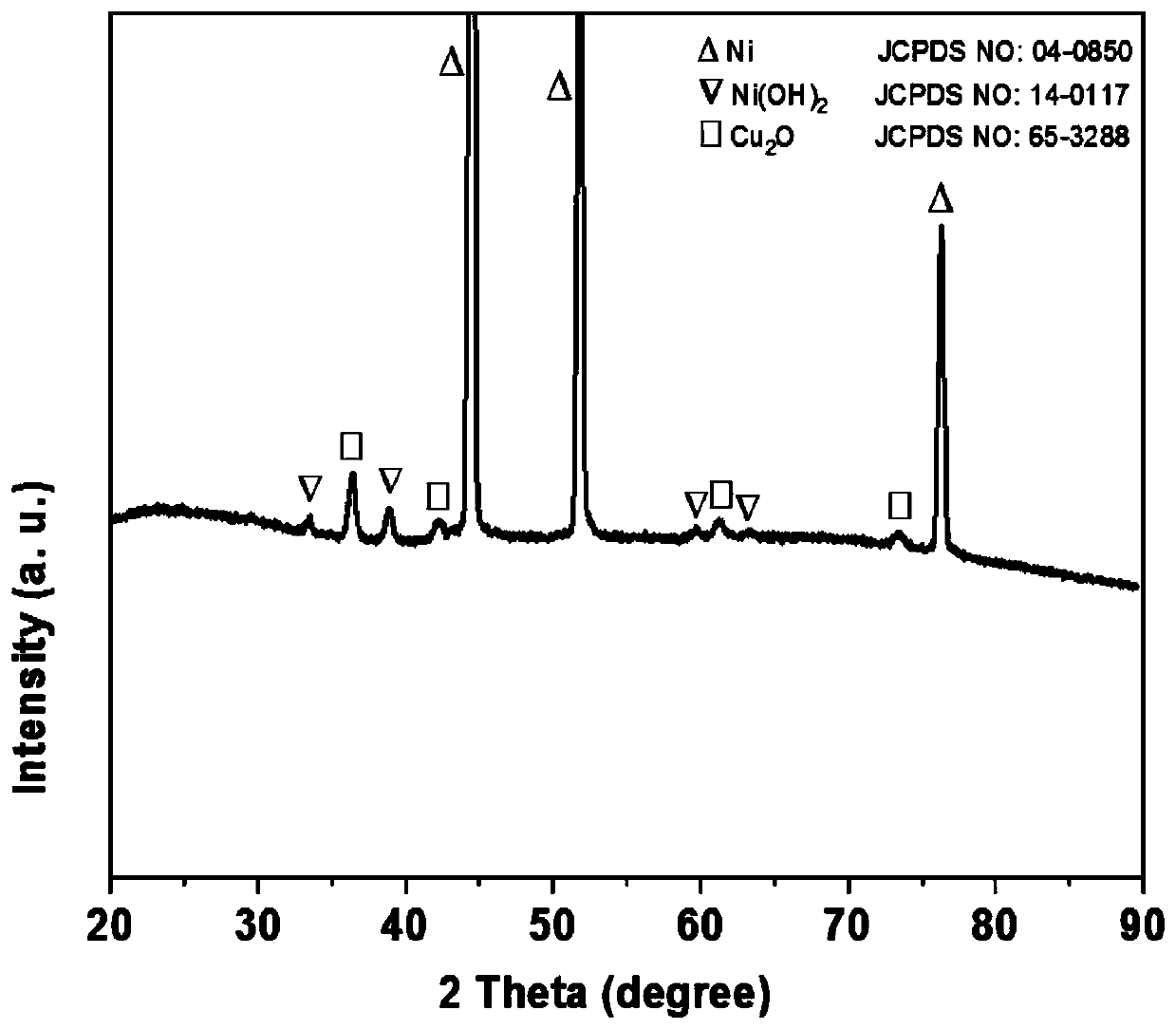
[0036] (2) Dissolve 0.5mmol of nickel nitrate hexahydrate, 2mmol of ammonium fluoride, and 5mmol of urea in 50mL of deionized water. After magnetically stirring for 15min, transfer the mixed solution into a 100mL reaction kettle, and then add 3cm× 5cm foam nickel; put the reaction kettle in a thermostat at 140°C, and react for 8 hours; cool down naturally, wash, and vacuum dry at 60°C to obtain Ni(OH) 2 / NF.
[0037] (3) Dissolve 0.3mmol of copper nitrate trihydrate, 2mmol of ammonium fluoride, and 5mmol of urea in 50mL of deionized water, and after magnetically stirring for 15min, transfer the mixed solution into a 100mL reactor, and then add the Ni in the above step. (OH) 2 / NF; put the reaction kettle in a thermostat at 140°C, and react for 12 hours; cool down naturally, wash, and dry in vacuum at 60°C to obtain Cu 2 O-Ni(OH) 2 / NF.
Embodiment 3
[0039] (1) Commercially available nickel foam is pretreated according to Example 1.
[0040] (2) Dissolve 1.0mmol of nickel nitrate hexahydrate, 2mmol of ammonium fluoride, and 5mmol of urea in 60mL of deionized water. After magnetically stirring for 15 minutes, transfer the mixed solution into a 100mL reaction kettle, and then add 3cm× 5cm foam nickel; put the reaction kettle in a 160°C incubator, and react for 15 hours; naturally cool down, wash, and vacuum-dry at room temperature to obtain Ni(OH) 2 / NF.
[0041] (3) Dissolve 0.5mmol copper nitrate trihydrate, 2mmol ammonium fluoride, and 5mmol urea in 60mL of deionized water, and after magnetic stirring for 15min, transfer the mixed solution into a 100mL reactor, and then add the Ni in the above step. (OH) 2 / NF; put the reaction kettle in a thermostat at 100°C, and react for 14 hours; cool down naturally, wash, and vacuum-dry at room temperature to obtain Cu 2 O-Ni(OH) 2 / NF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com