Use of protein kinase Czeta in regulating tumor cell chemotactic movement
A protein kinase and tumor cell technology, applied in the field of protein kinase Czeta to regulate tumor cell chemotaxis, can solve the problems of unseen design of specific anticancer drugs and unknown functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 EGF can induce chemotactic movement of MDA-231 cells
[0032] 1. Experimental method
[0033] Using a chemotaxis chamber to detect the chemotaxis movement of cells is a very mature method, which can be specifically operated using conventional methods in the art. Briefly, different concentrations of the chemokines EGF and SDF-1[alpha] were added to the wells of the bottom plate of the chemotaxis chamber. Separate the upper and lower chambers with a filter membrane, and add MDA-231 cells to the upper chamber. After incubation, the non-specific chemotaxis cells on the upper layer of the membrane were removed, and the membrane was fixed, stained with Diff-Quick, and counted under a microscope (400x field of view). The average number of 3 fields of view in each well was taken as the number of chemoattractant cells in the well. The ratio of the number of chemoattractant cells in the sample well to the number of cells in the control well is the chemotaxis index. ...
Embodiment 2
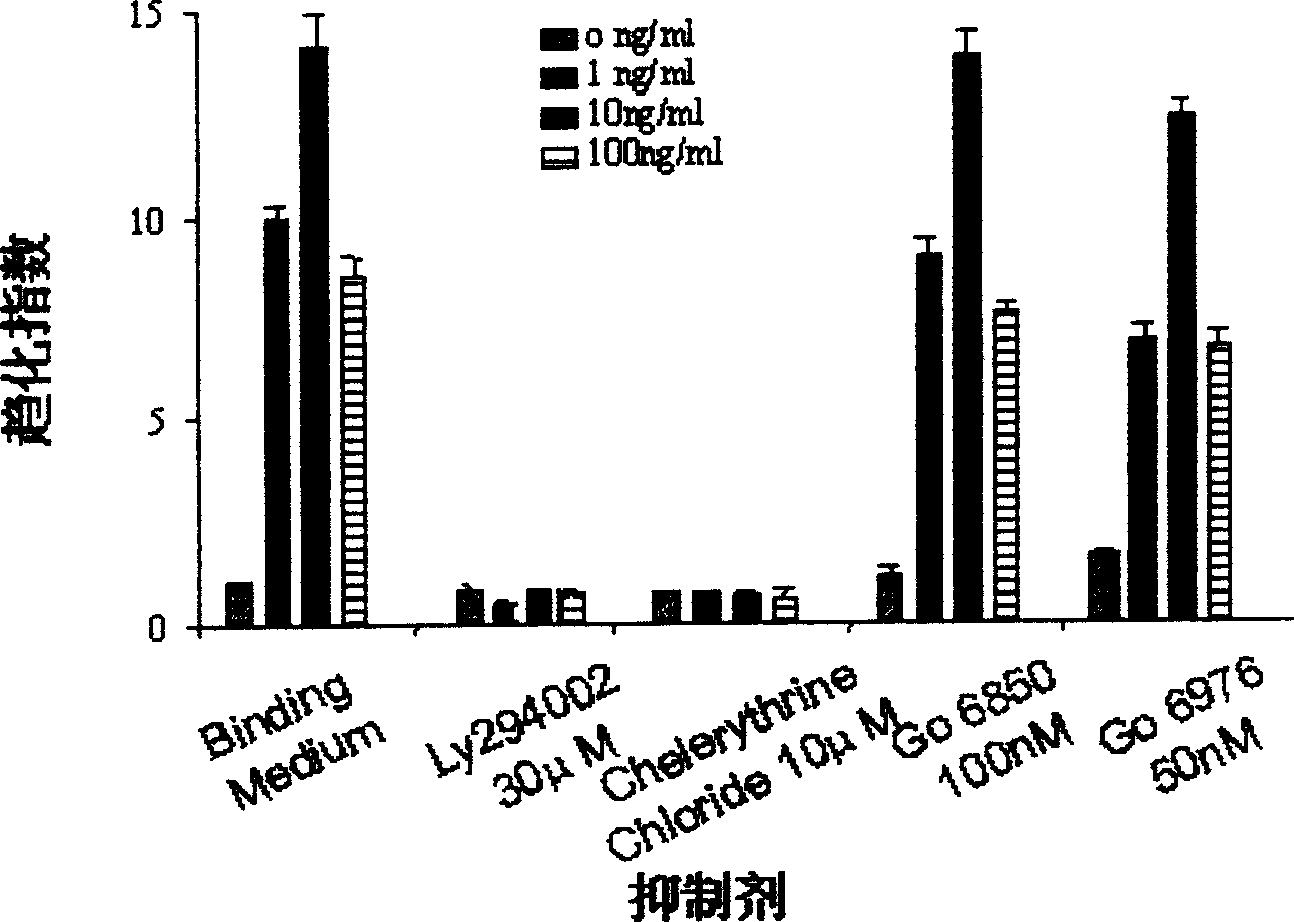
[0036] Example 2 Inhibition of PKCζ and PI3K Specific Inhibitors on MDA-231 Chemotaxis Movement
[0037] 1. Experimental method: pre-treat the cells with the specific PI3K inhibitor Ly294002 (30μM) and different PKC subtype-specific inhibitors, 100nM G6850, 50nM G6976, 10μM Chelerythrine Chloride at 37°C for 45-60min, then Add it directly to the upper plate hole of the chemotaxis chamber to detect the chemotaxis movement ability of the cells to EGF.
[0038] 2. Experimental results: see figure 1 , PI3K-specific inhibitor Ly294002 can effectively inhibit EGF-induced chemotaxis of MDA-231; similarly, PKCζ-specific inhibitor Chelerythrine Chloride can also completely inhibit the chemotaxis of MDA-231 cells. However, G6850 and G6976, which inhibit cPKC and nPKC, had no significant inhibitory effect on EGF-induced MDA-231 motility.
[0039] 3. Conclusion: Ly294002 and Chelerythrine Chloride can effectively inhibit the chemotaxis of EGF-induced breast cancer cell MDA-231, sugg...
Embodiment 3
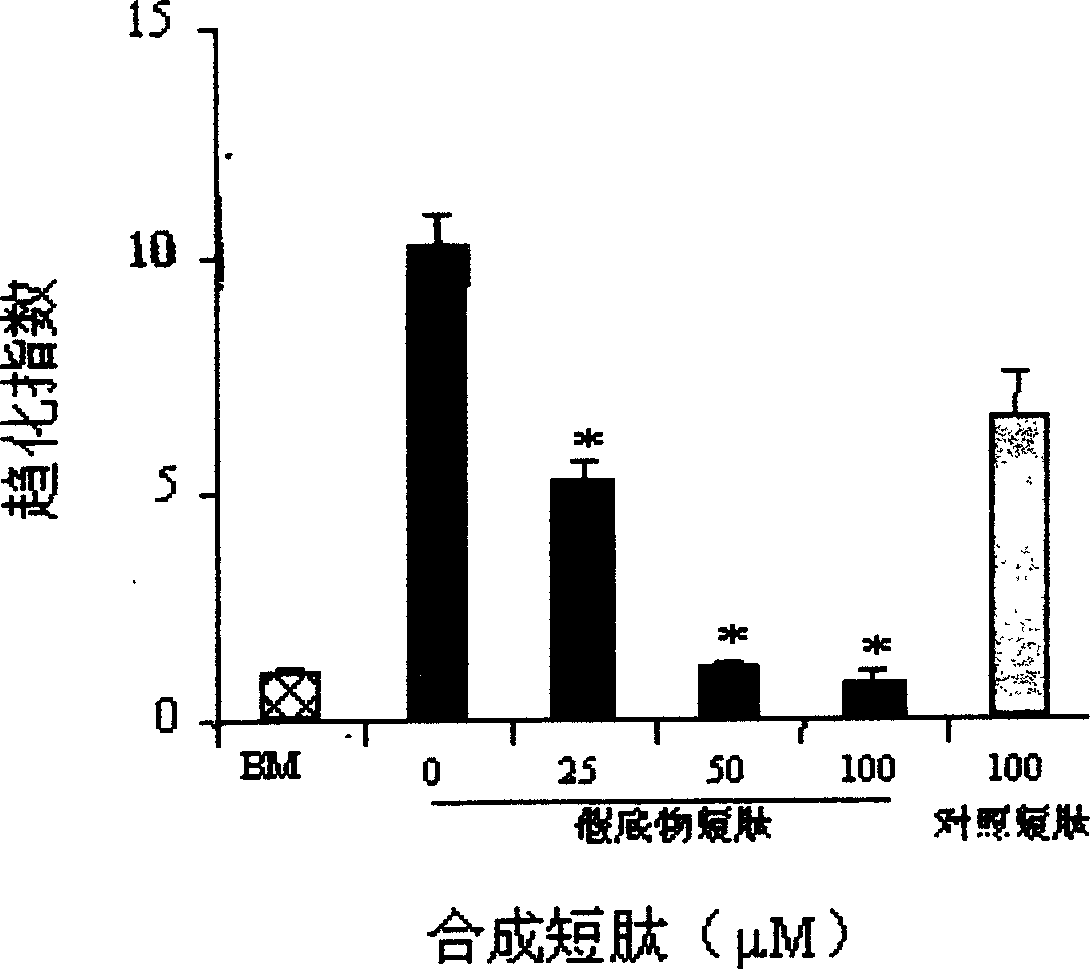
[0040] Example 3 Immunofluorescence method to determine whether EGF can specifically induce the activation of PKCζ molecule in MDA-231 cells
[0041] 1. Experimental method
[0042] MDA-231 cells were planted 48 hours before the experiment, and cultured for 3 hours with serum-free FPMI 1640 containing 0.1% BSA. The cells were continued to be treated with serum-free medium (FPMI 1640, 0.1% BSA) containing inhibitor (30 μM Ly294002) or without inhibitor for 60 min. Cells were fixed with 4% PFA after stimulation with medium (FPMI 1640, 0.1% BSA) or EGF (diluted to 50 ng / ml in FPMI 1640, 0.1% BSA) for a specified period of time. After washing twice with PBS, the membrane was permeated with 0.2% Tween 20 at 4°C for 30 min. After washing again, incubate with rabbit anti-human PKC specific polyclonal antibody (diluted 1:100 in PBS) at room temperature for 1 hour, wash thoroughly with PBS for 3 times, and then add FITC-(fluorescein isothiocyanate)-labeled goat anti-rabbit The secon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com