Medicine containing ketone effective component
A technology of active ingredients and medicines, applied in the field of medicines containing ketone active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Extraction of effective parts of Swertia japonica in eastern Sichuan.
[0036] Take the dried whole herb of Swertia chinensis and heat it twice with 6 times the amount of 95% ethanol under reflux, the first time is 2 hours, and the second time is 1 hour. Concentrate the extract, adjust the pH to 8 with NaOH, and let stand at 4--8°C for 1 hour, centrifuge, take the supernatant, concentrate to 0.5 times the volume of the medicinal material, and adjust the pH to 5 with HCl. 4-- After standing at 8°C for 12 hours, it was filtered. The effective part is obtained by drying the filter residue.
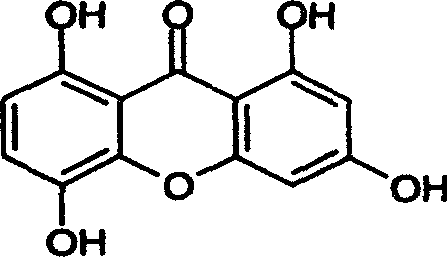
[0037] After analysis and identification, the content of ketone in the extract of the effective part is 63.6% based on demethyl gentianone.
Embodiment 2
[0039] The extract of the effective part prepared above was subjected to antibacterial experiment. The results are shown in Table 1.
[0040] Table 1
[0041] Bacteria name MIC MBC
[0042] Staphylococcus aureus 0.18 0.36
[0043] Shigella dysenteriae 0.09 0.18
[0044] Escherichia coli 0.18 0.36
[0045] Salmonella typhi 0.18 0.36
[0046] Note: The unit of numbers in the table is mg / ml
Embodiment 3
[0048] According to the methods disclosed in the literature, various ketones were isolated and used in antibacterial experiments, and compared with berberine. The results are shown in Table 2.
[0049]
[0050] Note: The unit of numbers in the table is: mg / ml
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com