N(2)-L-alanyl-L-glutamine aseptic powdery preparation and process for prepairing same
A technology of glutamine and sterile powder, applied in the direction of powder transportation, etc., can solve the problems of unqualified bacterial inspection, increase of preparation cost, increase of adverse reactions, etc., and achieve the effect of increasing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
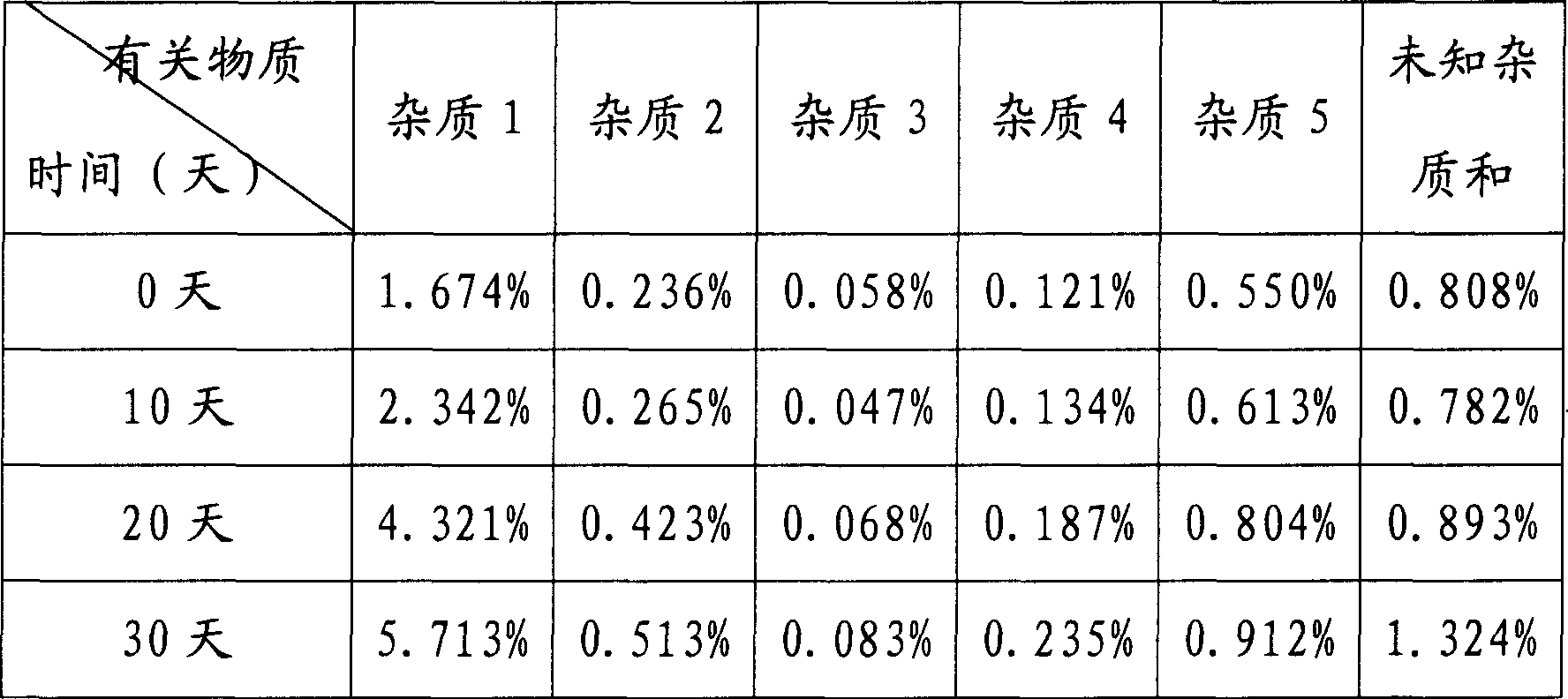
[0036] Under sterile conditions (the instruments and equipment used in the following operations have been aseptically treated), add 10kg of N(2)-L-alanyl-L-glutamine raw materials to 20kg of distilled water, stir and heat at 30-50°C, Dissolve, add 0.3kg of medical activated carbon, stir at this temperature for 20 minutes, filter out the activated carbon, filter the filtrate through a 0.22μm microporous membrane, sterilize the filtrate, stir at 30-50°C and add sterile ethanol dropwise to the filtrate until crystals are precipitated (Consumption of ethanol 20L), stirring slowly for 1.5 hours, slowly adding 17.14L of sterile ethanol dropwise within 1.5 hours at room temperature, stirring slowly for 2 hours after dropping, filtering the precipitated crystals, washing with an appropriate amount of cold ethanol, and drying under reduced pressure at 80°C to prepare Constant weight, 8.53 kg of white crystalline powder obtained.
Embodiment 2
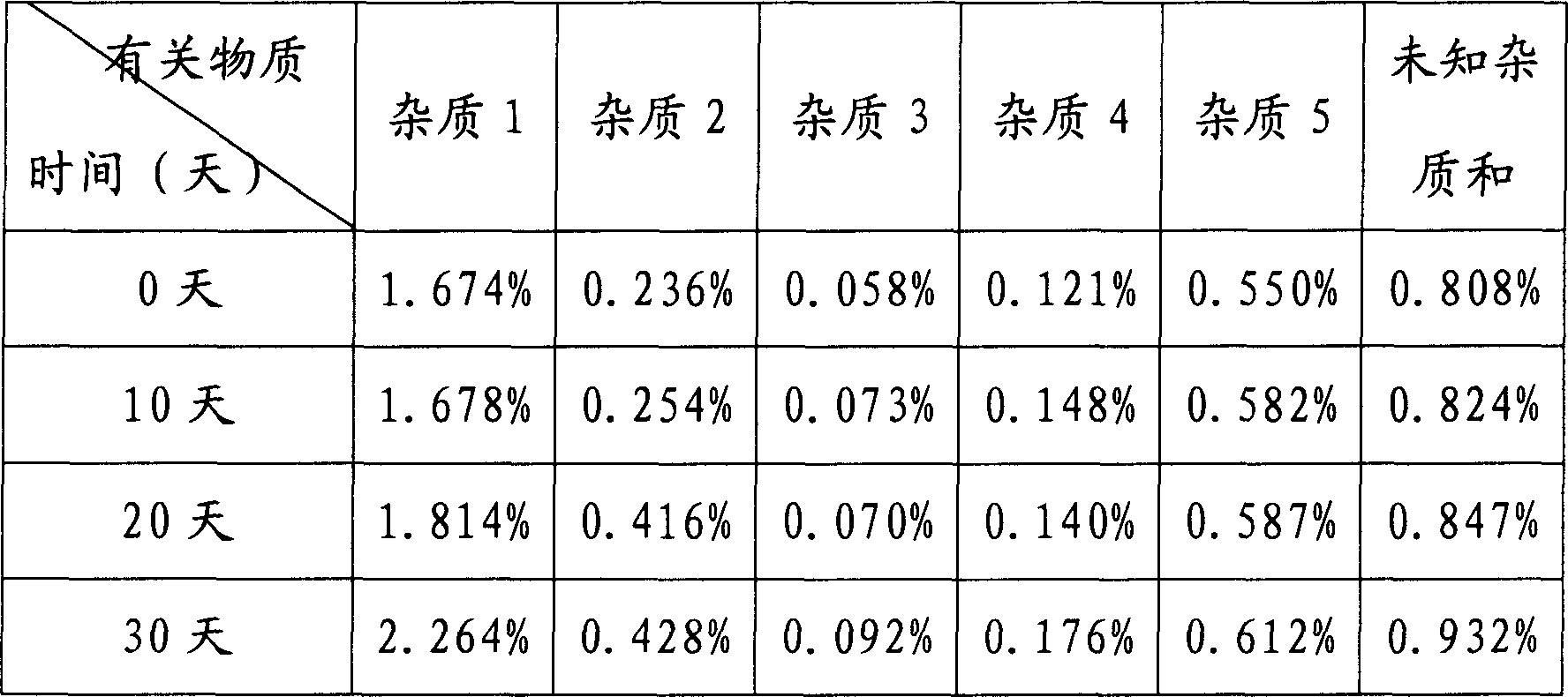
[0038] Under sterile conditions (the instruments and equipment used in the following operations have been aseptically treated), add 10kg of N(2)-L-alanyl-L-glutamine raw materials to 20kg of distilled water, stir and heat at 30-50°C, Dissolve, add 0.3kg of medical activated carbon, stir at this temperature for 20 minutes, filter out the activated carbon, filter the filtrate through a 0.22μm microporous membrane, sterilize the filtrate, stir at 30-50°C and add sterile ethanol dropwise to the filtrate until crystals are precipitated (Consumption of ethanol 20L), slowly stirred for 1.5 hours, slowly added 60L of sterile ethanol dropwise within 1.5 hours at room temperature, slowly stirred for 2 hours after dropping, filtered the precipitated crystals, washed with an appropriate amount of cold ethanol, and dried under reduced pressure at 80°C to obtain a constant Weight, the obtained white crystalline powder 9.26 kg.
Embodiment 3
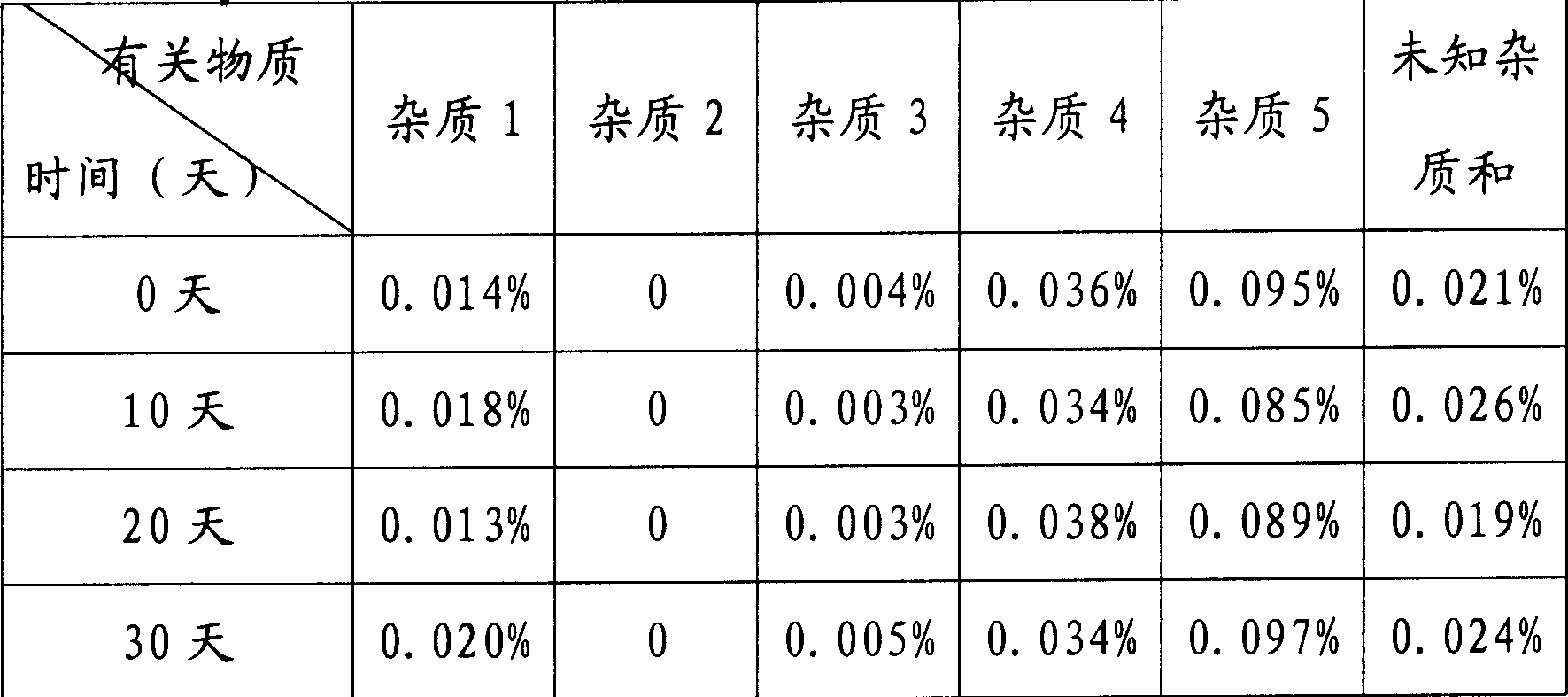
[0040] Under sterile conditions (the instruments and equipment used in the following operations have been aseptically treated), add 10kg of N(2)-L-alanyl-L-glutamine raw materials to 20kg of distilled water, stir and heat at 30-50°C, Dissolve, add 0.3kg of medical activated carbon, stir at this temperature for 20 minutes, filter out the activated carbon, filter the filtrate through a 0.22μm microporous membrane, sterilize the filtrate, stir at 30-50°C and add sterile ethanol dropwise to the filtrate until crystals are precipitated (Consumption of ethanol 20L), stir slowly for 1.5 hours, slowly add 160L of sterile ethanol dropwise within 1.5 hours at room temperature, slowly stir for 2 hours after dropping, filter the precipitated crystals, wash with an appropriate amount of cold ethanol, and dry under reduced pressure at 80°C to obtain a constant Weight, the obtained white crystalline powder 9.28 kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com