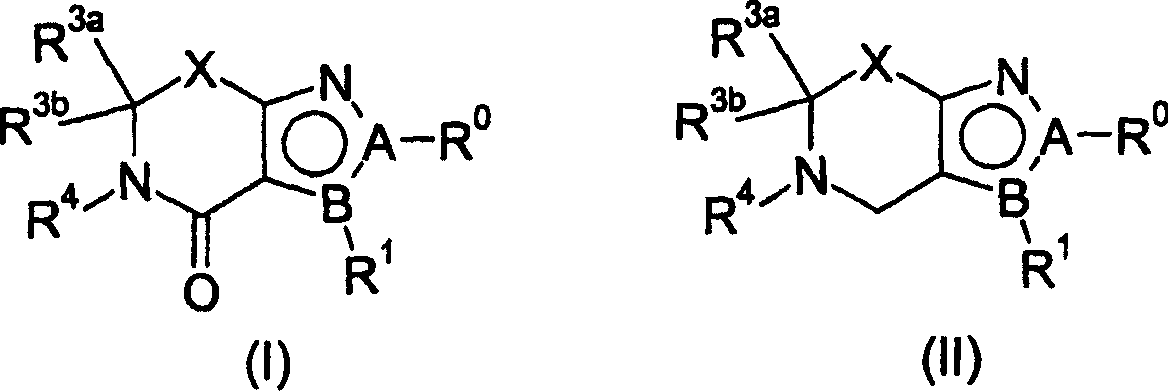
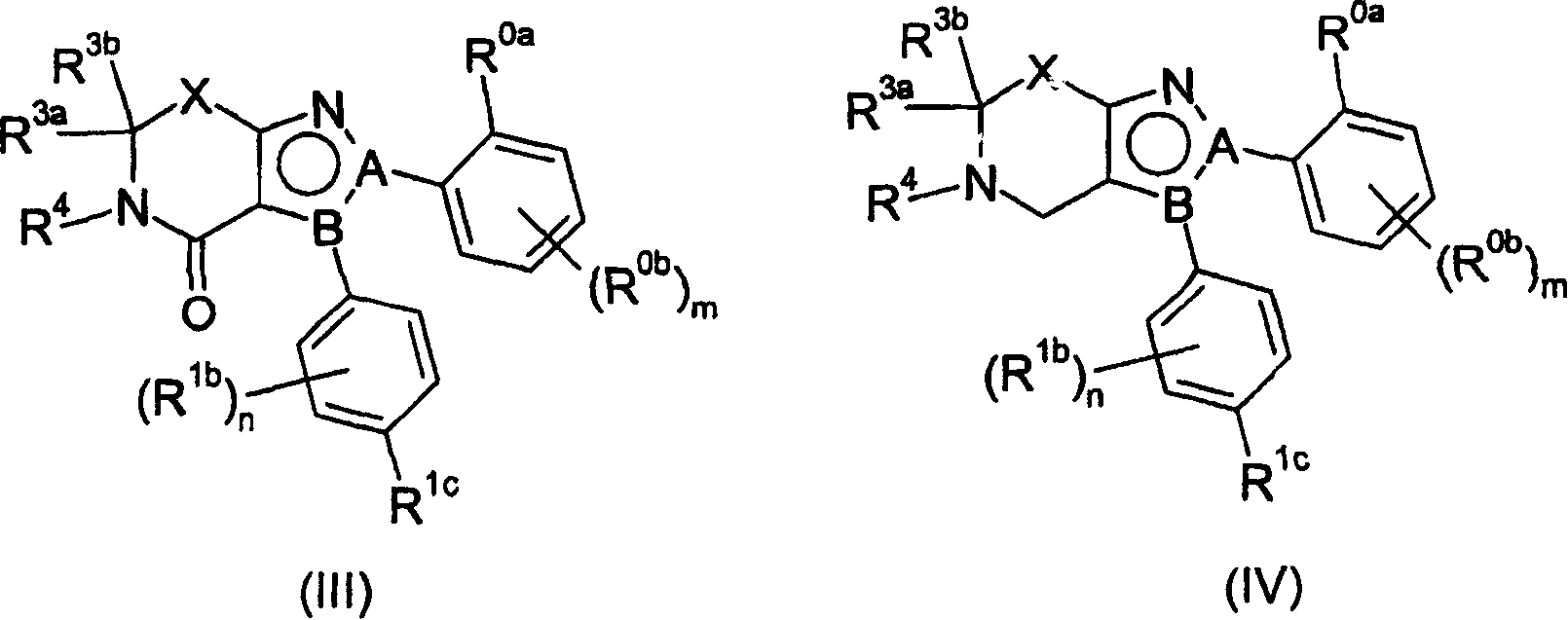
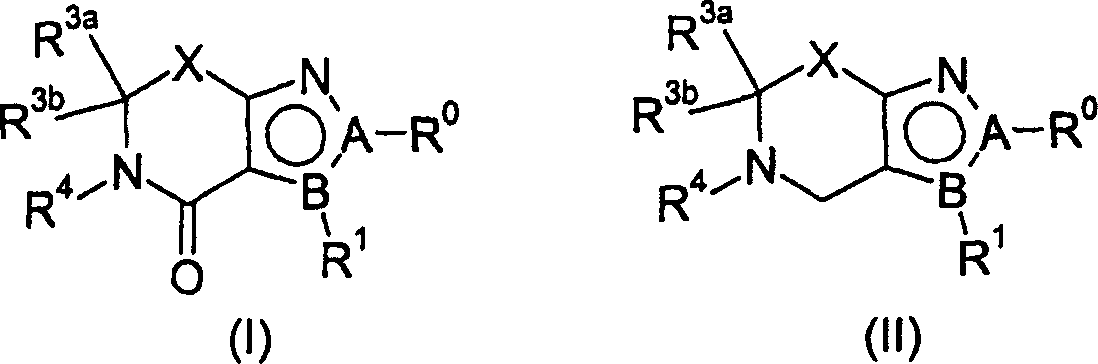
Cannabinoid receptor ligands and uses thereof
A halogen, compound technology, applied in diseases and/or disorders, CB1 receptor antagonists, treatment of diseases regulated by cannabinoid receptor antagonists, can solve problems such as liver toxicity in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] 2-(2-Chloro-phenyl)-5-isopropyl-3-(4-methoxy-phenyl)-5,6-dihydro-2H-pyrrolo[3,4-c]pyr Preparation of oxazol-4-one (1A-1):
[0179]
[0180] 2-(2-Chloro-phenyl)-3-iodo-5-isopropyl-5,6-dihydro-2H-pyrrolo[3,4-c]pyrazol-4-one purged with nitrogen I-1a (50 mg), cesium fluoride (38 mg), 3-methoxyphenylboronic acid (74 mg) and 1,2-dimethoxy The solution in ethane (1 mL) was stirred at 100°C in a sealed vial for 7 hours. The reaction was cooled and partitioned between ethyl acetate / water. The organic phase was dried (Na 2 SO 4 ), and concentrated in vacuo to give an oil. Silica gel chromatography (25% to 35% ethyl acetate: hexane) gave the title compound (1A-1) as an off-white solid, 27 mg. in CDCl 3 (ppm) in
[0181] h 1 NMR: δ7.56-7.40 (m, 6H), 6.80 (d, 2H), 4.63 (m, 1H), 4.38 (br d, 2H), 1.25 (d, 6H); ms (LCMS) m / z = 382.3 (M+1).
[0182] Using methods similar to those described above for the synthesis of compound 1A-1, the following compounds listed in Tab...
Embodiment 2
[0191] Using methods similar to those described above for the synthesis of compound 1A-1 and outlined in Scheme II above, using suitable starting materials either commercially available or prepared using formulations well known to those skilled in the art, the following Compounds listed in Table 2A.
[0192] Table 2A
[0193]
[0194]
Embodiment 3
[0196] Compounds were synthesized using a method similar to that described above for 1A-1 The following compounds listed in Table 3A were prepared using the appropriate starting materials, either commercially available or prepared using formulations well known to those skilled in the art, as outlined in Scheme IV above.
[0197] Table 3A
[0198]
[0199]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com