Sol containing titanium dioxide, thin film formed therefrom and production process of the sol
A technology of titanium dioxide and sol, applied in the direction of titanium dioxide, chemical instruments and methods, catalyst activation/preparation, etc., can solve problems such as difficult to use photocatalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] (1-1.) Synthesis of titanium dioxide sol
[0237] Distilled water (908 mL) was placed in a reactor equipped with a reflux condenser and heated steadily at 95°C. An aqueous solution of titanium tetrachloride (Ti content: 16.5% by mass, specific gravity: 1.52, product of Sumitomo Titanium) (92 mL) was added dropwise to the reactor at about 1 mL / min while maintaining stirring at about 200 rpm. Care was taken to prevent the temperature of the reaction mixture from dropping. The titanium tetrachloride concentration of the reaction mixture was found to be 0.5 mol / L (4% by mass in terms of titanium dioxide). In the reactor, the reaction mixture became cloudy immediately after starting the addition of titanium tetrachloride, but the temperature was maintained. When the addition was complete, the temperature was raised to 101°C (near boiling temperature) and held for 60 minutes. The sol thus obtained was washed with pure water through an ultrafiltration membrane (Microza ACP-...
Embodiment 2
[0265] (2-1.) Synthesis of a titanium dioxide-containing sol by dissolving a transition metal compound in a raw material
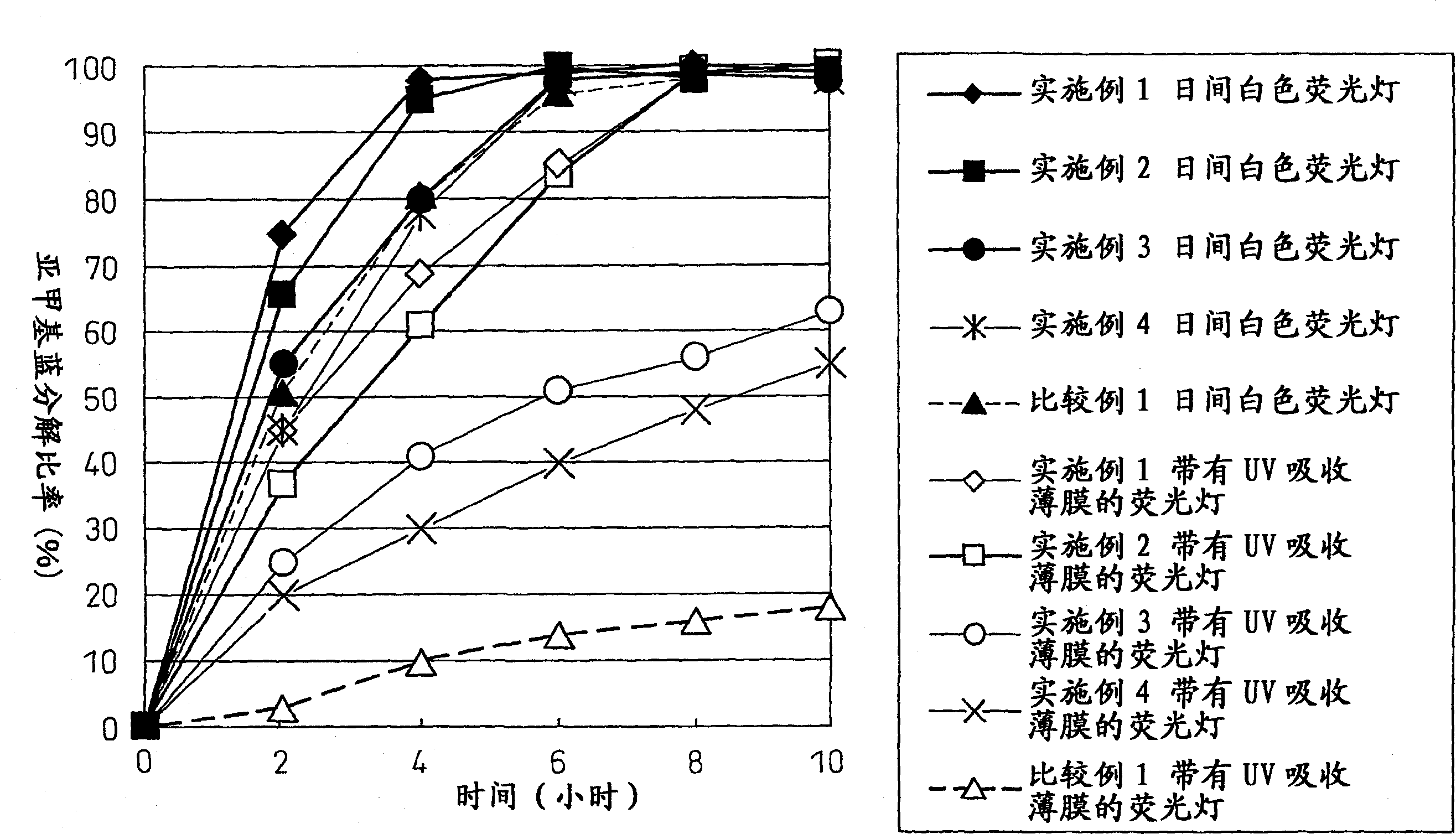
[0266] Hexachloroplatinic acid hexahydrate (0.108 g, 0.1% by mass based on titanium dioxide converted into platinum) was dissolved in an aqueous solution of titanium tetrachloride (92 mL), to thereby prepare an aqueous solution of titanium tetrachloride containing platinum. The procedure of (1-1.) was repeated except that the solution thus prepared was used instead of the titanium tetrachloride aqueous solution to thereby synthesize the sol, thereby synthesizing the sol. Thus, a sol containing a transition metal compound and titanium dioxide is obtained in a single step.
[0267] The amount of the precipitated component was found to be 3% by mass of the total solid content of the sol. The transmittance of the sol measured at 550 nm by using a measuring cell with an optical path length of 2 mm was 68%.
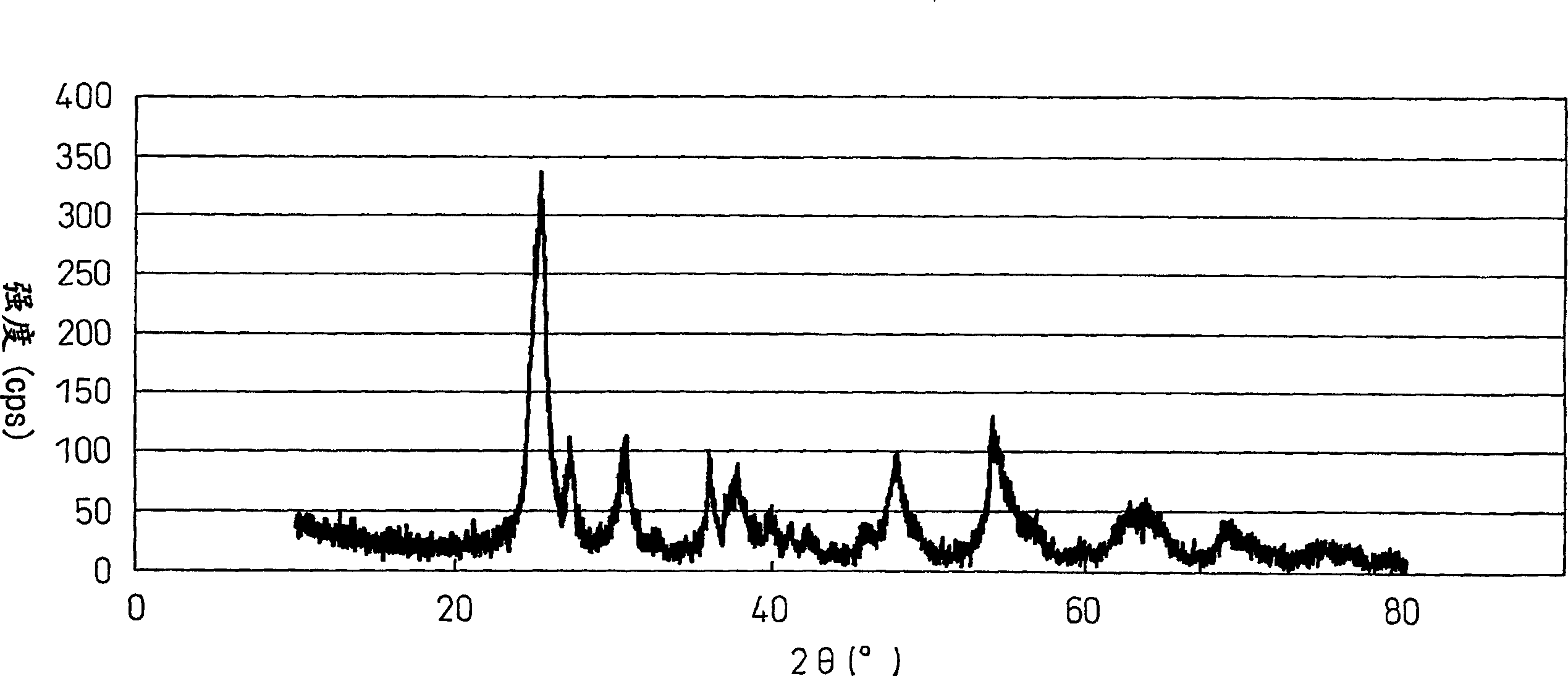
[0268] The BET specific surface area of the solid t...
Embodiment 3
[0281] (3-1.) Synthesis of titanium dioxide sol
[0282] The procedure of (1-1.) was repeated to thus synthesize a brookite-containing titania sol.
[0283] (3-2.) Mixing of titanium dioxide sol and transition metal (Fe) compound
[0284] The steps of (1-2.) were repeated except that hexachloroplatinic acid hexahydrate (special grade, Kanto Kagaku) was replaced by ferric chloride (special grade, product of Kanto Kagaku) (0.097 g, 0.1% by mass based on titanium dioxide converted into iron). preparation) (0.054 g) to thereby mix the titanium dioxide sol with the metal compound.
[0285] The amount of the precipitated component was found to be 3% by mass of the total solid content of the sol. The transmittance of the sol measured at 550 nm by using a measuring cell with an optical path length of 2 mm was 69%.
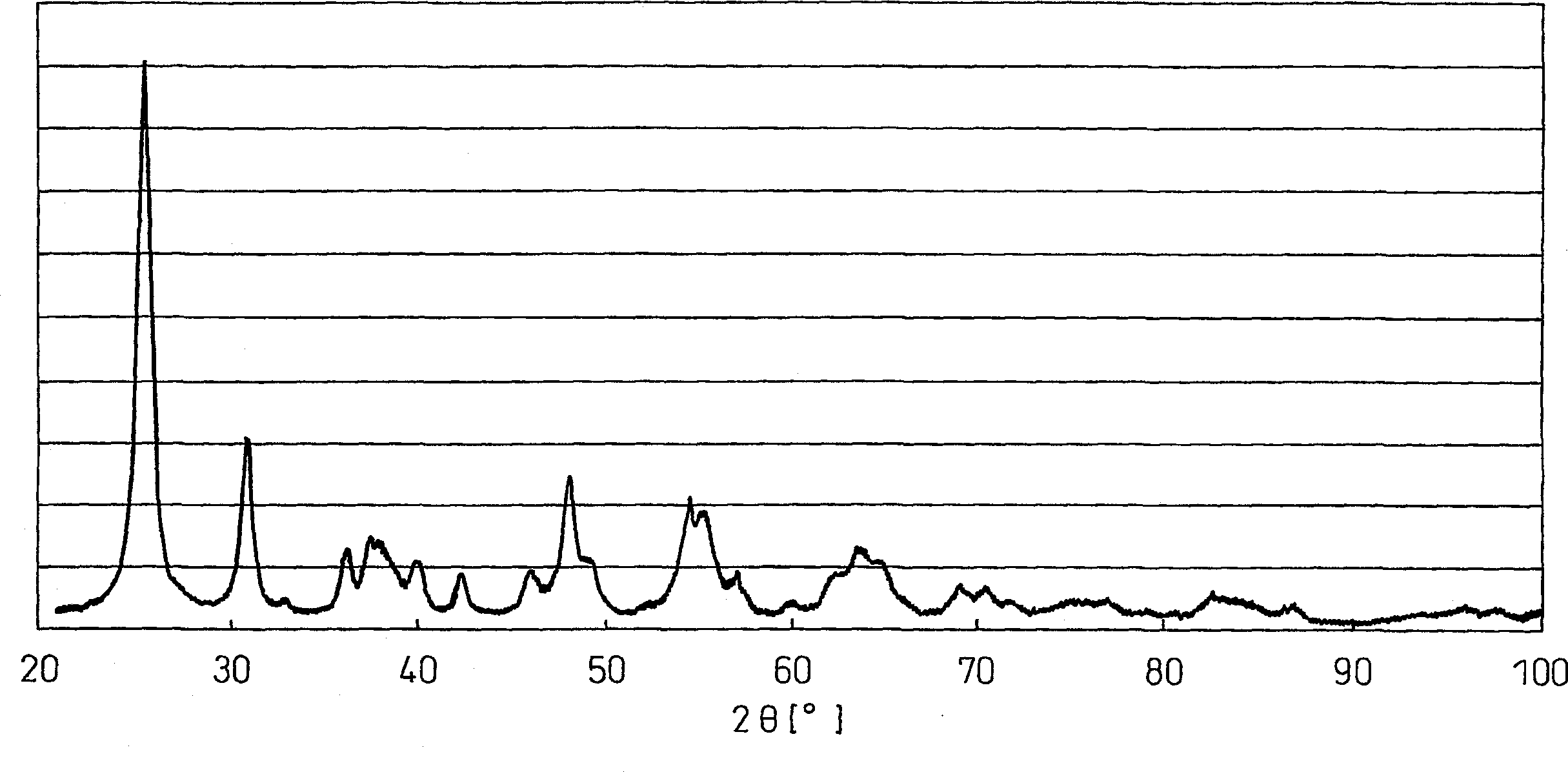
[0286] The solid sample thus obtained was subjected to BET specific surface area measurement, powder X-ray diffraction measurement and Rietveld analysis. The resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap