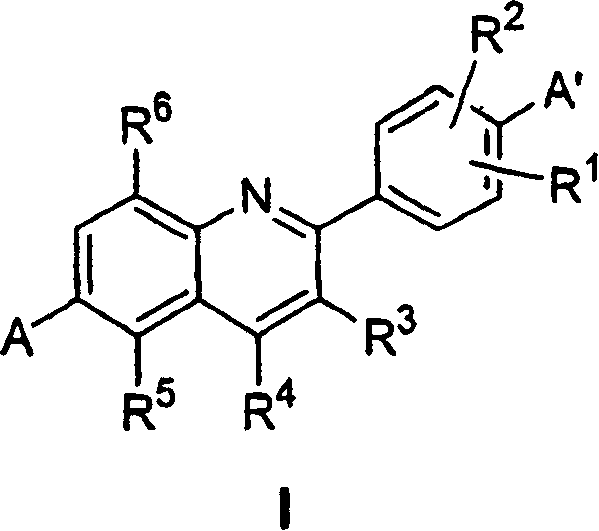
Phenyl quinolines and their use as estrogenic agents
A technology of quinoline and phenyl, which is applied in the field of phenylquinoline compounds, can solve problems such as unexplained cell-specific effects of the same compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Method A: Preparation of ethyl substituted benzoyl acetates 1.
[0106] A typical procedure for the preparation of ethyl (3-fluoro-4-methoxybenzoyl)acetate (1b) is described
[0107] Add NaH (60% dispersion in mineral oil, 42 g, 1.05 mol, 3 equiv, washed with hexane) and CO(OEt) via an addition funnel under reflux under nitrogen. 2 (85mL, 0.70mol, 2eq) in 1,4-dioxane (300mL) was added dropwise to a suspension of 3-fluoro-4-methoxyacetophenone (58.9g, 0.350mol) in 1,4- Dioxane (150 mL) solution. After the addition was complete, the mixture was stirred at reflux for an additional 30 min. After cooling to room temperature, glacial acetic acid was slowly added to acidify the reaction mixture, and the solvent was removed under reduced pressure. Water was then added and the mixture was extracted with EtOAc. Organic layer water, NaHCO 3 washed with aqueous solution, brine, and dried (Na 2 SO 4 ), filtered and concentrated to obtain pure crude product as a yellow oil, wh...
Embodiment 1a
[0110] Ethyl (4-methoxybenzoyl)acetate (1a).
[0111] This compound was prepared by Method A using 4-methoxyacetophenone. Yellow oil; Yield: 95%;
[0112] 1 H-NMR (300MHz, CDCl 3 ): δ1.26(t, J=7.3Hz, 3H), 3.88(s, 3H), 3.94(s, 2H), 4.21(q, J=7.3Hz, 2H), 6.95(m, 2H), 7.93 (m, 2H). Minortautomer: 1.33(t, J=7.3Hz, 3H), 3.86(s, 3H), 4.27(q, J=7.3Hz, 2H), 5.57(s, 1H), 6.92(m, 2H), 7.74 (m, 2H), 12.65 (s, 1H); MS (ESI) m / z 223 ([M+H] + );
[0113] Elemental Analysis, C 12 h 14 o 4 Theoretical values: C: 64.85, H: 6.35. Found values: C: 64.92, H: 6.11.
Embodiment 1b
[0115] Ethyl (3-fluoro-4-methoxybenzoyl)acetate (1b).
[0116] This compound was prepared according to Method A. Yellow oil; Yield: 94%;
[0117] 1 H-NMR (300MHz, CDCl 3 )δ1.26(t, J=7.1Hz, 3H), 3.93(s, 2H), 3.97(s, 3H), 4.21(q, J=7.1Hz, 2H), 7.01(dd, J=8.4, 8.5 Hz, 1H), 7.72(m, 2H). Minor tautomer: 1.33(t, J=7.1Hz, 3H), 3.94(s, 3H), 4.28(q, J=7.1Hz, 2H), 5.57(s, 1H), 6.99(t, J=8.4Hz, 1H), 7.54(m, 2H), 12.62(s, 1H); MS(ESI) m / z 241([M+H] + );
[0118] Elemental Analysis, C 12 h 13 FO 4 Theoretical values: C: 60.00, H: 5.45. Found values: C: 60.12, H: 5.25.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com