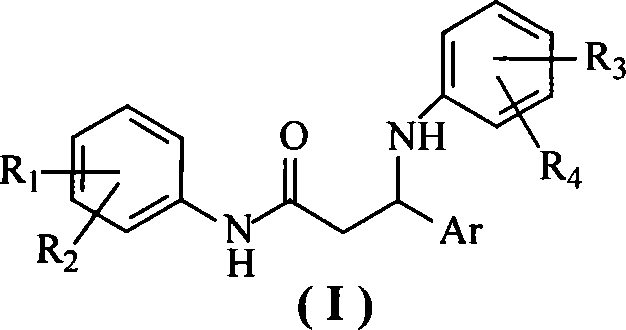
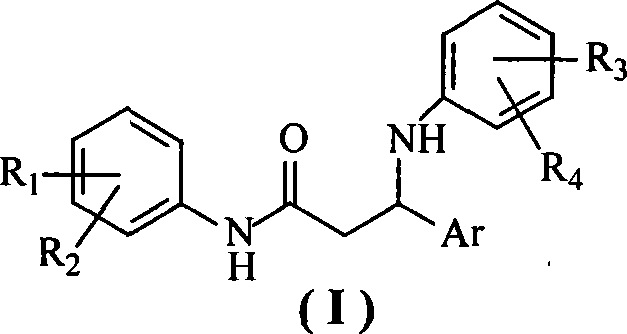
N-aryl-beta-aryl amidine-propionamide compounds, preparation method and use thereof
A technology of propionamide and compound, applied in the field of medicinal chemistry, can solve problems such as liver toxicity, gastrointestinal discomfort, blurred vision, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1N-(4-nitrophenyl)-3-phenyl-3-(4-chloroanilino)-propionamide
[0028]
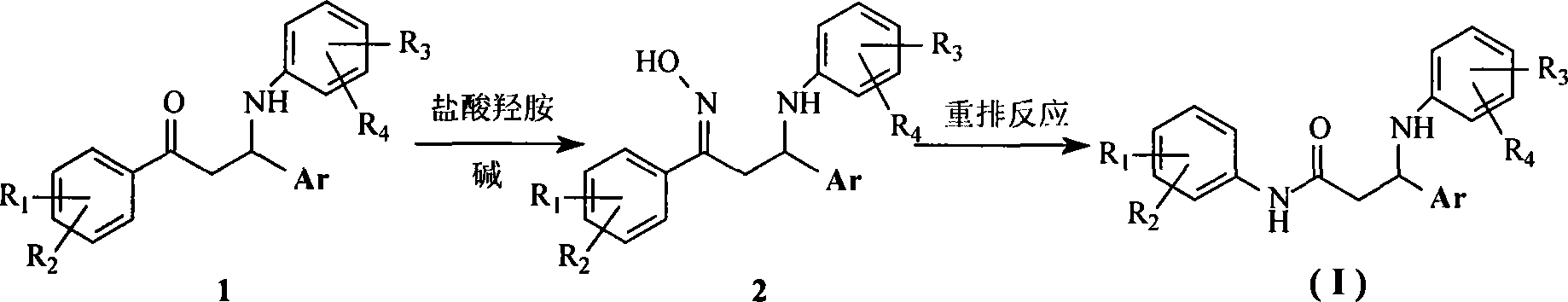
[0029] Add 1.9 g (5 mmol) of 3-phenyl-3-(4-chloroanilino)-1-(4-nitrophenyl)-1-propanone and 20 ml of ethanol into the reaction flask, stir well, then add hydrochloric acid in sequence 0.56 grams (8 mmol) of hydroxylamine, 1.1 grams (8 mmol) of potassium carbonate and 10 ml of deionized water were heated to reflux and stirred for 5 hours. After the reaction was completed, stand at room temperature, filter the precipitated solid, and recrystallize with ethanol / water to obtain 1- (4-nitrophenyl)-3-phenyl-3-(4-chloroanilino)-1-acetone oxime yellow crystal 1.41 g, yield 70.91%; 1 H-NMR (400MHz, CDCl 3 )δ: 8.19(d, 2H, J=9.2Hz), 7.66(d, 2H, J=9.2Hz), 7.36~7.21(m, 5H), 7.00(d, 2H, J=8.8Hz), 6.39( d, 2H, J=8.8Hz), 4.67(t, 1H, J=5.2Hz), 3.67~3.58(m, 1H), 3.08(dd, 1H, J 1 =13.2Hz,J 2 =5.2Hz); ESI-MS m / z: 418.8 (M + +Na).
[0030] In a dry round-bottomed flask, add 4.5 mmo...
Embodiment 2
[0031] The preparation of embodiment 2N-(4-methylphenyl)-3-(2-thienyl)-3-(4-chloroanilino)-propionamide
[0032]
[0033] The operation process is the same as in Example 1, except that 1-(4-nitrophenyl)-3-phenyl-3-(4-chloroanilino)-1-acetone is used with 1-(4-methylphenyl)- 3-(2-thienyl)-3-(4-chloroanilino)-1-propanone was substituted to obtain a light yellow powder solid with a yield of 42.6%; mp161~163°C; 1 H-NMR (400MHz, CDCl 3 )δ: 7.41(s, 1H), 7.25(d, 4H, J=8.8Hz), 7.09(d, 2H, J=8.8Hz), 7.20~6.92(m, 3H), 6.62(d, 2H, J =8.8Hz), 5.13(t, 1H, J=6Hz), 2.94(d, 2H, J=6Hz), 2.30(s, 3H); IR (KBr tablet): v NH 3406.51,v NH 3356.05,v =CH 3069.80, v C-H 2918.47, v. C=O 1661.17, beta NH 1597.01, v. C=C 1503.03, delta CH2 1437.70, # C=C 896.43-680.00; ESI-MS m / z: 371 (M + +H), 393(M + +Na).
Embodiment 3
[0034] The preparation of embodiment 3N-(4-methylphenyl) 3-phenyl-3-(4-chloroanilino)-propionamide
[0035]
[0036] The operation process is the same as in Example 1, except that 1-(4-nitrophenyl)-3-phenyl-3-(4-chloroanilino)-1-acetone is used with 1-(4-methylphenyl)- Substituted by 3-phenyl-3-(4-chloroanilino)-1-propanone, a white powder solid was obtained with a yield of 21.2%; mp176~178°C; 1 H-NMR (400MHz, CDCl 3 )δ: 7.37~7.25(m, 5H), 7.19(d, 2H, J=8.0Hz), 7.08(d, 2H, J=8.4Hz), 7.04(d, 2H, J=9.2Hz), 6.53( d, 2H, J=8.8Hz), 4.81(t, 2H, J=5.2Hz), 2.86(m, 2H), 2.29(s, 3H); IR (KBr tablet): v NH 3409.49, v NH 3247.84, v =CH 3062.83, v C-H 2922.36, v C=O 1655.34, beta NH 1599.75, v. C=C 1498.86, delta CH2 1453.34, # C=C 813.88-676.65; ESI-MS m / z: 365 (M + +H), 388(M + +Na).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com