Phenylindole compound and preparation method and application thereof
A kind of compound and alkyl technology, applied in phenylindole compound and its preparation and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
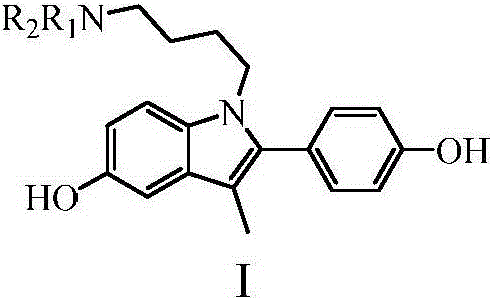
[0035] Example 1: Preparation of 1-[4-(dimethylamino)butyl]-5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
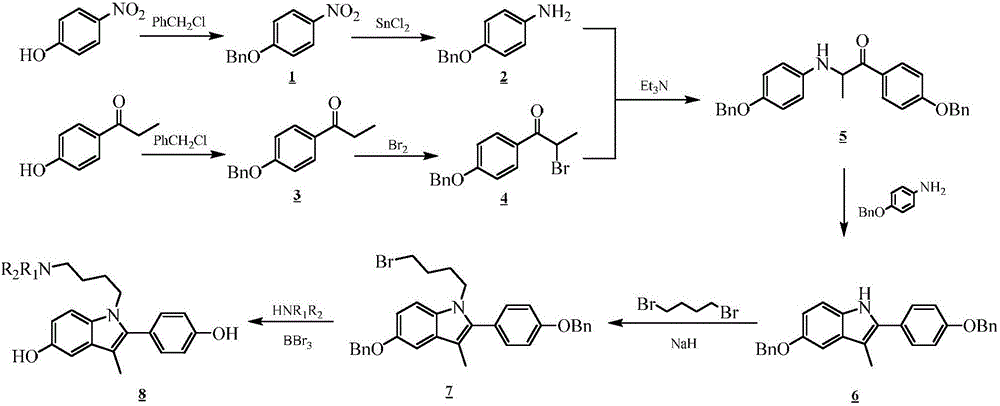
[0036] Step A): Preparation of 4-nitrobenzyloxybenzene
[0037] Put 4-nitrophenol (20.0g, 0.152mol) and anhydrous potassium carbonate (131.1g, 0.950mol) in a 1000mL flask, use acetone as solvent, add dropwise benzyl chloride (12.0g, 0.095mol), and reflux reaction After 24 hours, the solvent was evaporated under reduced pressure, the residue was stirred with water, filtered with suction, and dried to obtain 19.8 g of a light yellow solid with a yield of 90.8%. m.p.105-106°C. ESI-MS: m / z139 ([M+H] + ).
[0038] Step B): Preparation of 4-benzyloxyaniline
[0039] Put 4-nitrobenzyloxybenzene (10.0g, 0.044mol) and tin protochloride (50g, 0.220mol) in a 1000mL round bottom flask, ethanol as solvent, N 2 Under protection, react at 45°C for 24h. Evaporate the solvent under reduced pressure, transfer the residue to a 1000mL beaker with 600mL of sodium carbonate solut...
Embodiment 2
[0052] Example 2: Preparation of 1-[4-(diethylamino)butyl]-5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
[0053] According to the method of Example 1, 0.39 g of a yellow solid was obtained with a yield of 38.9%. m.p.102-103°C. ESI-MS: m / z367 ([M+H] + ). 1 H-NMR (400MHz, DMSO-d 6 )δ9.67(s,1H),8.68(s,1H),7.24(d,J=8.7Hz,1H),7.19(d,J=8.5Hz,2H),6.90(d,J=8.5Hz, 2H), 6.79(d, J=2.2Hz, 1H), 6.64(dd, J 1 =8.6Hz,J 2 =2.3Hz,1H),3.98(t,J=6.4Hz,2H),3.34(m,6H),2.05(s,3H),1.5(d,J=5.1Hz,4H),1.87(m,6H ).
Embodiment 3
[0054] Example 3: Preparation of 1-[4-(isopropylamino)butyl]-5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
[0055] According to the method of Example 1, 0.30 g of a yellow solid was obtained with a yield of 52.1%. m.p.116-117°C. ESI-MS: m / z353 ([M+H] + ),375([M+Na] + ). 1 H-NMR (400MHz, DMSO-d 6 )δ9.05(s,1H),8.12(s,1H),7.25(d,J=8.6Hz,1H),7.17(d,J=8.4Hz,2H),6.88(d,J=8.4Hz, 2H), 6.81(d, J=2.2Hz, 1H), 6.64(dd, J 1 =8.7Hz,J 2 =2.3Hz,1H),4.09(s,1H),3.97(t,J=6.8Hz,2H),3.52(m,1H),2.06(s,2H),2.04(s,3H),1.54(m ,4H), 1.24(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com