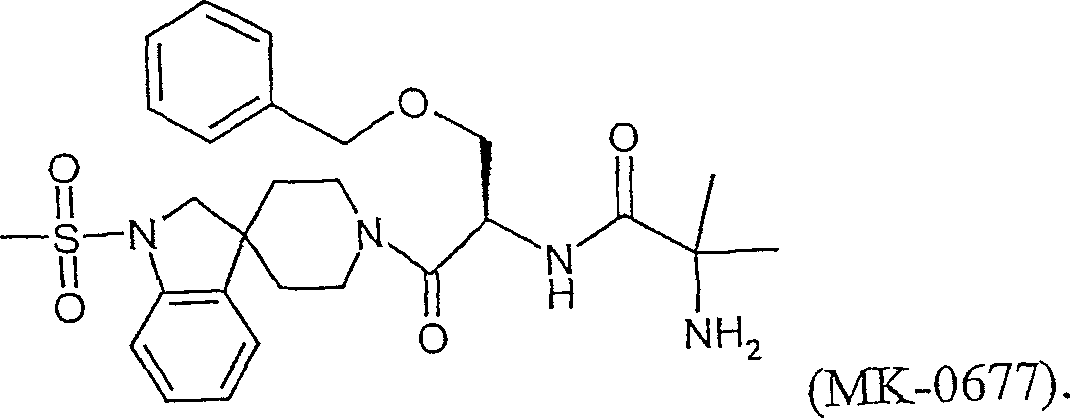
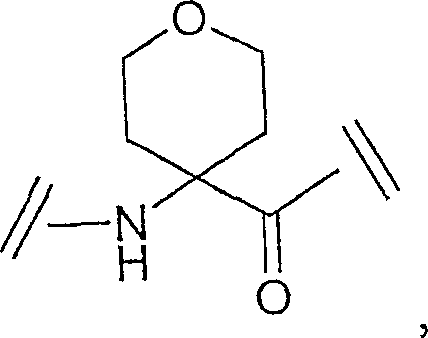
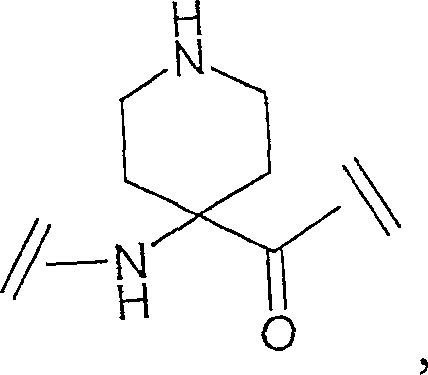
Ghrelin analogs
A compound, -A1-A2-A3-A4-A5-A6-A7-A8-A9-A10-A11-A12-A13-A14-A15-A16-A17-A18-A19-A20-A20-A21-A22 - The technology of A23-A24-A25-A26-A27-A28-R1 is applied in the direction of hormone peptides, animal/human proteins, peptide/protein components, etc., and can solve the problem of low oral bioavailability and stimulation of peptidyl growth hormone secretagogue Non-peptide compound research and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0882] Embodiment 1: (Glu 3 (O-hexyl))hGhrelin(1-28)-NH 2
[0883] The title peptide was synthesized on an Applied Biosystems (Foster City, CA) Model 433A Peptide Synthesizer. 4-(2',4'-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxyacetamide-norleucyl-MBHA resin (Rink Amide MBHA resin) with 0.72mmol / g substituent was used , Novabiochem, San Diego, CA). Fmoc amino acids (AnaSpec, San Jose, CA) with the following side chain protections were used: Fmoc-Arg(Pbf)-OH, Fmoc-Pro-OH, Fmoc-Gln-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc )-OH, Fmoc-Ala-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Val-OH, Fmoc-His(Trt)-OH, Fmoc-Phe-OH, and Fmoc-Asp(OtBu)-OH. Boc-Gly-OH (Midwest Bio-Tech, Fishers, IN) was used in position 1. N-α-Fmoc-L-glutamic acid γ-4-{N-(1-(4,4-Dimethyl-2,6-dioxocyclohexylene)-3-methylbutyl)-amino } Benzyl ester (Fmoc-Glu(ODmab)-OH) (Chem-Inpex International, WoodDale, IL) was used at position 3. Synthesis was performed on a 0.25 mmol scale. The Fmoc group was removed by treatment...
Embodiment 2
[0885] Embodiment 2: (Aib 2 )hGhrelin(1-28)-NH 2
[0886] According to the synthesis described in Example 1 (Glu 3 (O-hexyl))hGhrelin(1-28)-NH 2 The title peptides were synthesized by the method described above, except for the following: Fmoc-Ser-OH for position 3, Fmoc-Aib-OH for position 2, and Boc-Gly-OH for position 1. After assembly of the peptide chains, the peptide-resin was treated with 25% piperidine in DMF for 3 x 2 h. The resin was washed with DMF and treated with octanoic acid (2.5 mmol, 10-fold excess), HBTU (2.2 mmol), HOBt (2.2 mmol) and DIEA (7.5 mmol) in DMF for 2 h. The resin was washed with DMF and treated with octanoic acid (2.5 mmol), DIC (2.5 mmol), HOBt (2.5 mmol) and DMAP (0.025 mmol) in DMF for 2 h. The final cleavage and purification steps are the same as in Example 1. Analytical HPLC determined that the product was homogeneous with a purity of 99% and a yield of 18.5%.
[0887] The molecular weight of the surface product analyzed by electrospr...
Embodiment 3
[0888] Embodiment 3: (Glu 3 (NH-hexyl))hGhrelin(1-28)-NH 2
[0889] The title peptide was synthesized on an Applied Biosystems (Foster City, CA) Model 430A Peptide Synthesizer modified for accelerated Boc-chemical solid-phase peptide synthesis, see Schnolzer, et al., Int. J. Ppetide Protein Res., 40:180 (1992 ). 4-Methylbenzhydrylamine (MBHA) resin (Peninsula, Belmont, CA) with 0.91 mmol / g substituent was used. Boc amino acids (Midwest Bio-Tech, Fishers, IN; Novabiochem, San Diego, CA) with the following side chain protections were used: Boc-Ala-OH, Boc-Arg(Tos)-OH, Boc-His(DNP)-OH , Boc-Val-OH, Boc-Leu-OH, Boc-Gly-OH, Boc-Gln-OH, Boc-Lys(2ClZ)-OH, Boc-Ser(Bzl)-OH, Boc-Phe-OH, Boc -Glu(OcHex)-OH and Boc-Pro-OH. Position 3 in the residue sequence used Fmoc-Glu(OtBu)-OH (Novabiochem, San Diego, CA). Synthesis was performed on a scale of 0.25 mmol. Treat with 100% TFA for 2×1 min to remove the Boc group. Boc amino acids (2.5 mmol) were preactivated with HBTU (2.0 mmol) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com