Methods of dissociating nongenotropic from genotropic activity of steroid receptors
A steroid, non-genetic technology used in biochemical equipment and methods, receptors/cell surface antigens/cell surface determinants, analytical materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0208] Regulation of osteoblast and osteocyte programming by estrogens and androgens in vivo and in vitro
[0209] Death
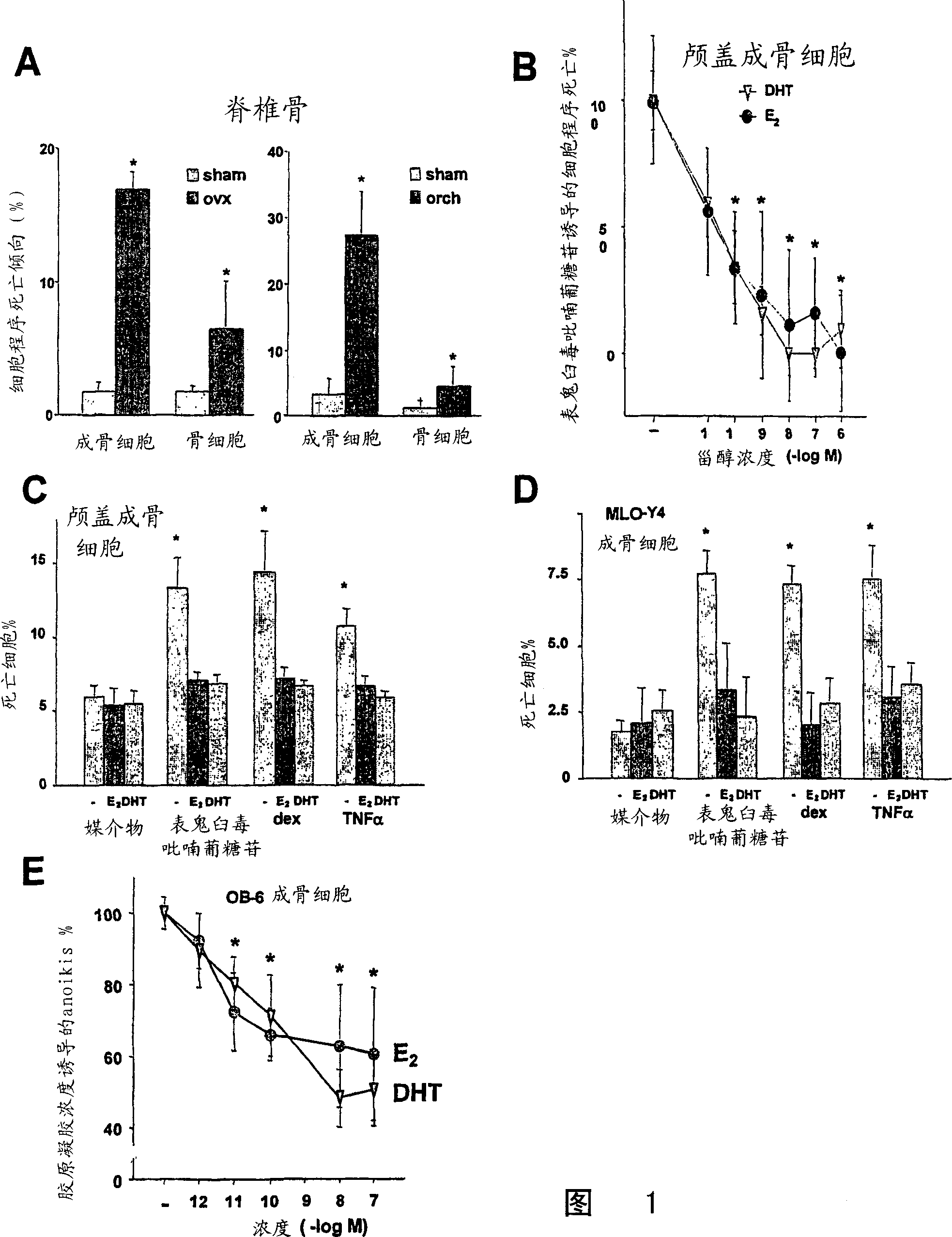
[0210] Compared with sham-operated controls, spinal osteoblast calvarial cell apoptosis was increased 10-fold and 8.3-fold, while bone cell apoptosis was increased 4-fold and 3.5-fold in ovariectomized or orchiectomized mice (Fig. 1A). After taking 17β-estradiol (E 2 ) in ovariectomized mice (unpublished data). To illustrate the mechanism of this effect, primary calvarial cell cultures and bone cell lines were used as in vitro models. E. 2 Or 5α-dihydrotestosterone (DHT) can inhibit the apoptosis induced by epipodophyllotoxin glucopyranoside in calvaria cells in a dose-dependent manner, and the maximum effect appears at 10 -9 -10 -8 M concentrations (Fig. 1B); the same results were obtained with MLO-Y4 cells (unpublished). When induced with any of the following three different pro-apoptotic stimuli, E 2 or DHT inhibited apop...
example 2
[0213] ER (α or β) or AR can transmit anti-apoptotic signals with equal efficiency, while
[0214] Regardless of whether the ligand is an estrogen or an androgen.
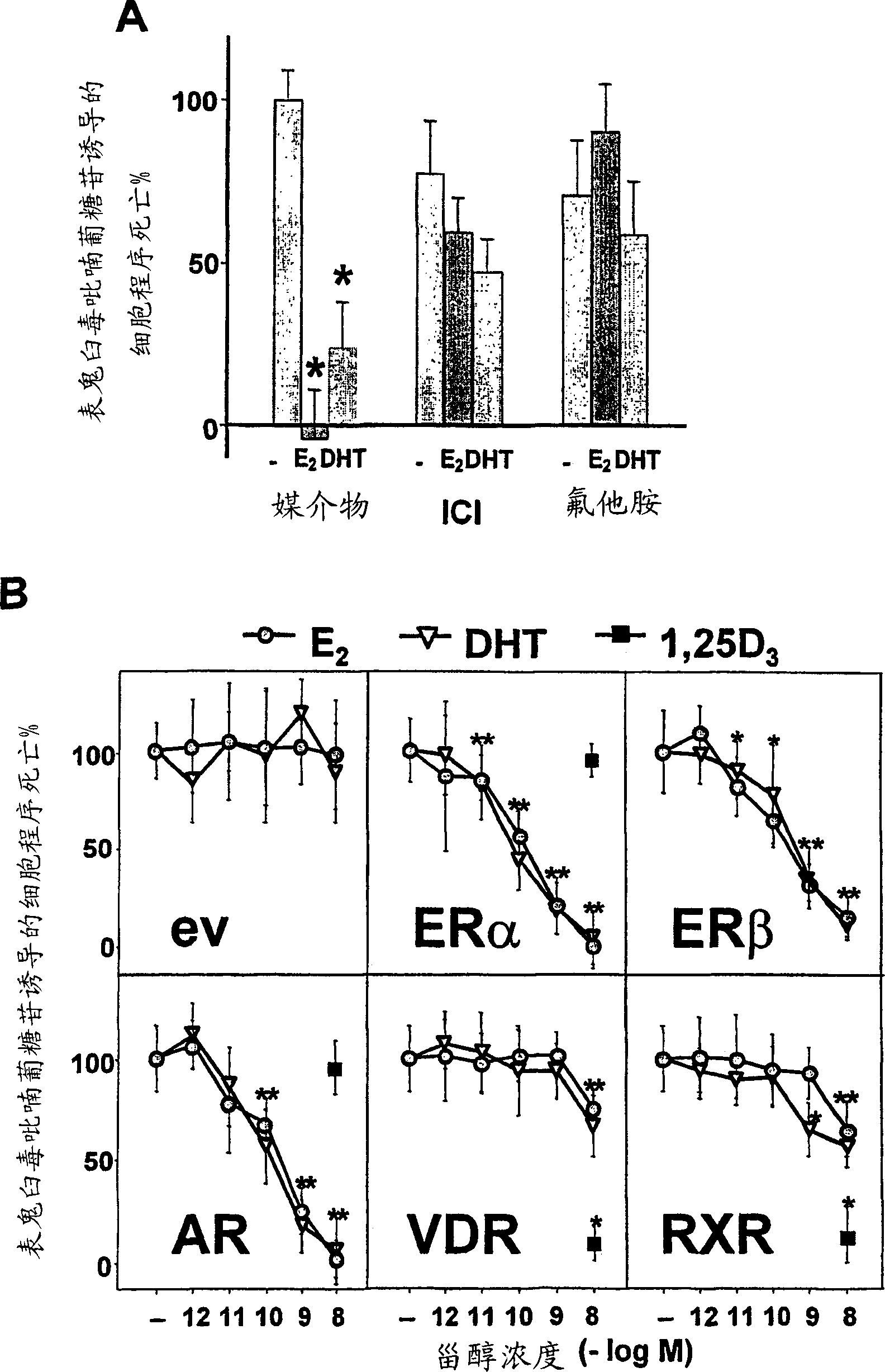
[0215] The estrogen receptor antagonist ICI182780 or the androgen receptor antagonist flutamide can inhibit E 2 The anti-apoptotic effects of DHT and DHT strongly suggest that the effects of the sex steroids are mediated through ER and AR.
[0216] Surprisingly, however, E 2 The anti-apoptotic effect of DHT can also be eliminated by flutamide, and the effect of DHT can be eliminated by ICI182780 ( figure 2 A). Consistent with this unexpected result, antihormones including ICI182780 have previously been shown by others to interact with various types of steroid receptors (Nawaz, Z., 1999, Cancer Res. 59, 372-376).
[0217] In order to determine E 2 The dependence of the anti-apoptotic effects of DHT and DHT on ER and AR, and to dissect the role of each receptor in the unexpected results obtained w...
example 3
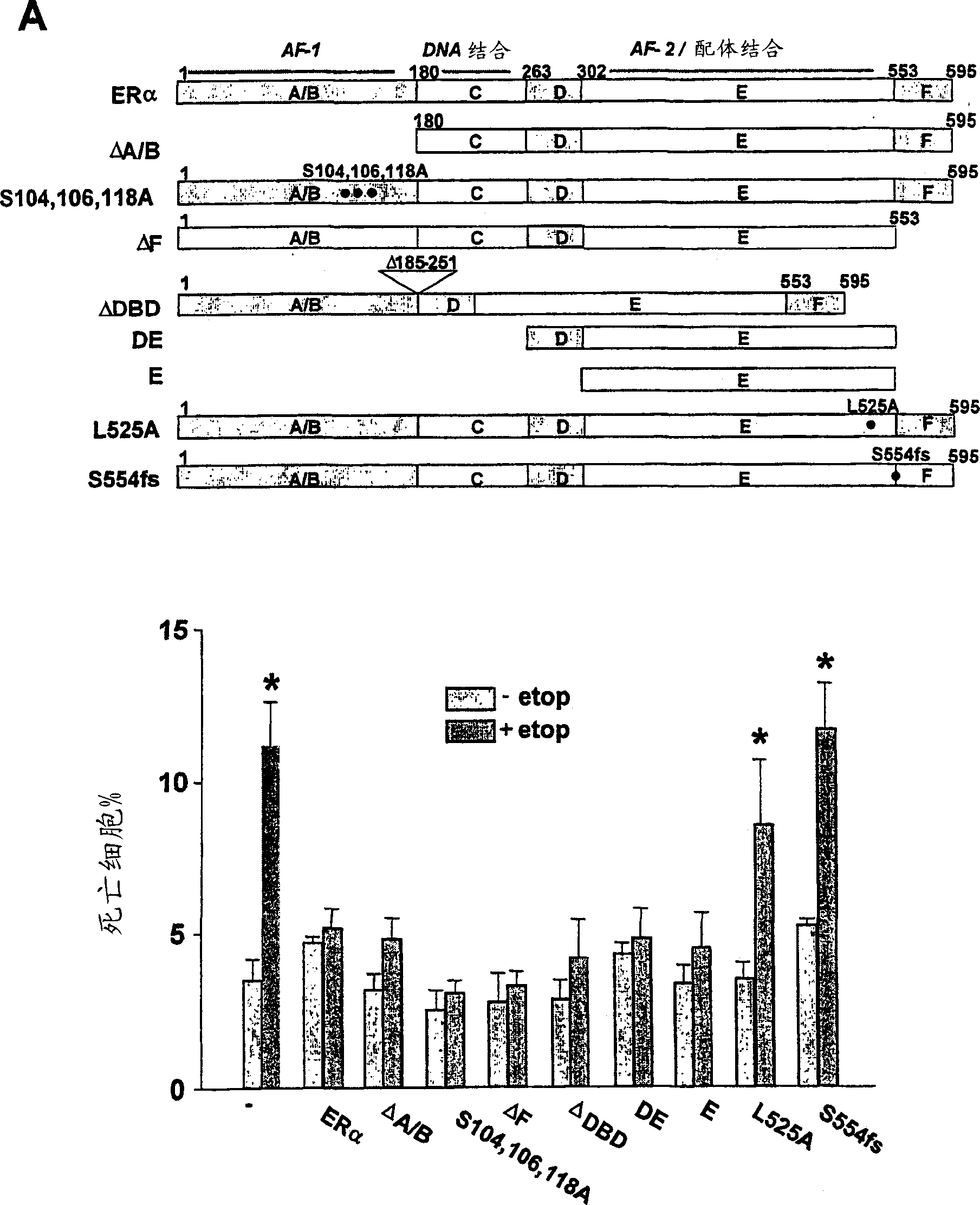
[0220]The anti-apoptotic activity of ERα is located in the E (ligand binding) domain and requires
[0221] Extranuclear localization of the protein
[0222] Using ERα as a prototype, it was determined whether the domain required for anti-apoptotic activity of the sex steroid receptor is the same as that required for transcriptional activity ( Figure 3A ). The effects of several mutants of ERα on inhibiting apoptosis were compared. Some of these mutants have been disclosed previously and have been shown to produce the expected protein when transiently transfected into human cells such as the HeLa cells used in the present study (Schodin, D.J. et al., 1995, J.Biol.Chem.270, 31163-31171; Kraus, W.L.et al., 1997, J SteroidBiochem.Mol.Biol.63, 175-188; Ekena, K.et al., 1996, J.Biol.Chem. 271, 20053-20059; Ekena, K. et al., 1997, J. Biol. Chem. 272, 5069-5075; Montano et al., 1995, Mol. Endocrinol. 9, 814-825). In unpublished experiments, it has been found that m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com