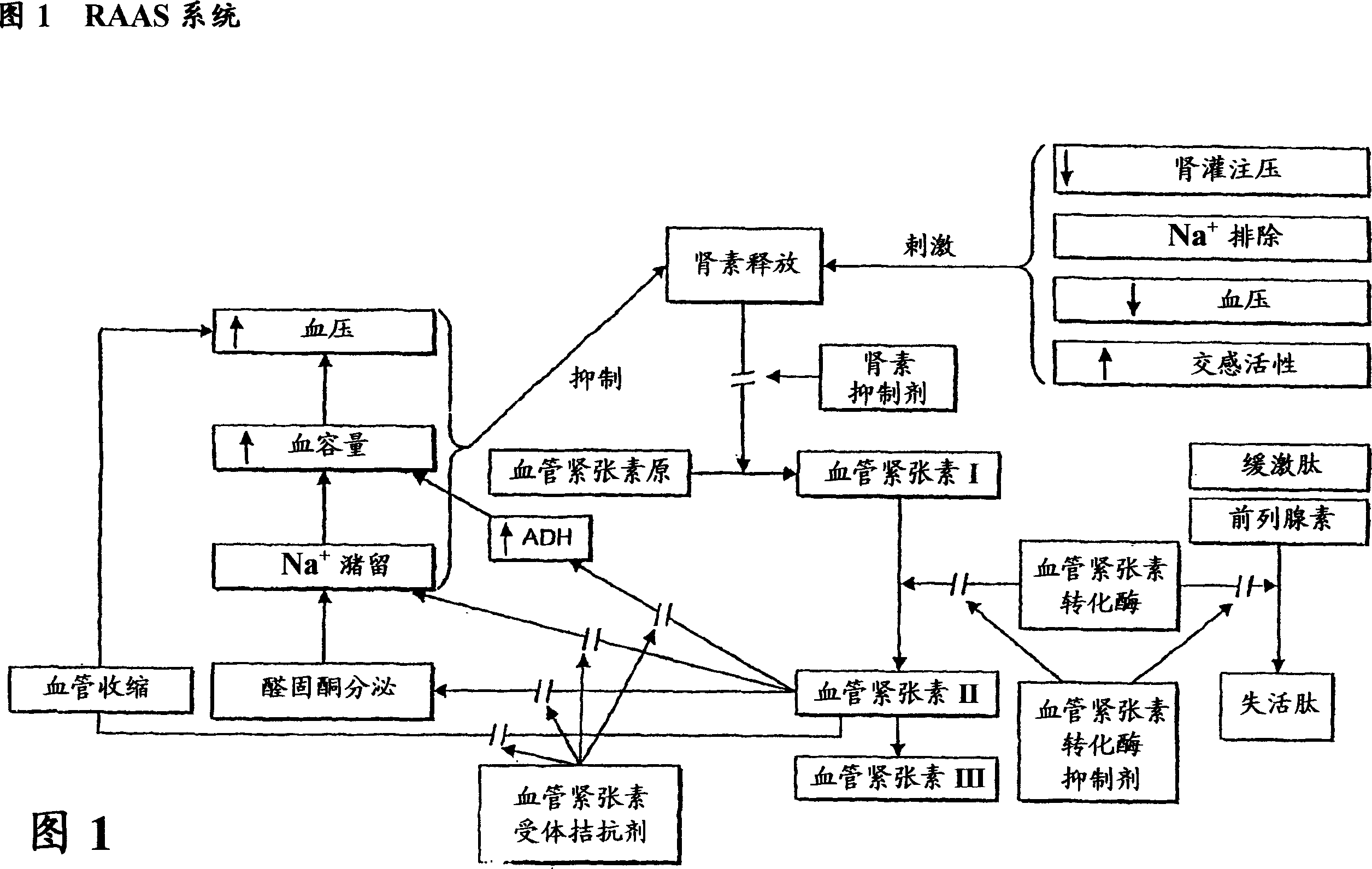
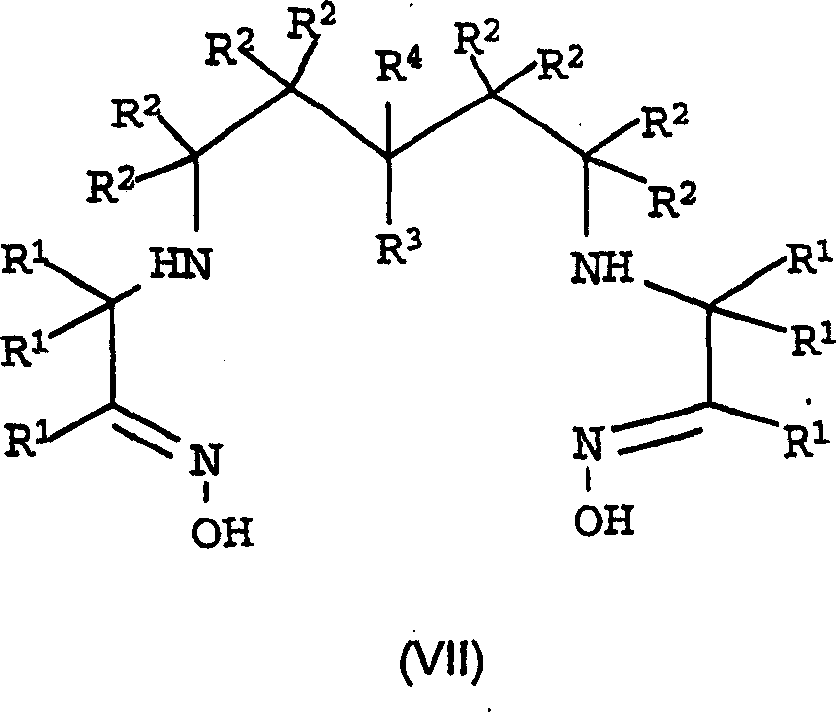
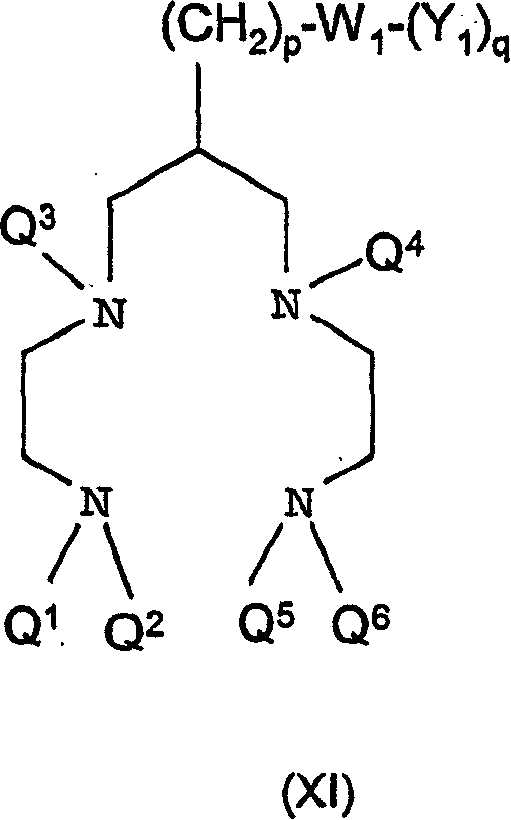
Conjugates of angiotensin ii and an imaging moiety
An imaging and drug technology, which is applied in the fields of new drugs for effective monitoring and treatment, and new pharmaceutical compositions and precursors for monitoring treatment, preparation of diagnostic agents, and can solve problems such as arrhythmia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] Example 1 Ang II analogs with optical imaging dyes
[0191] Fluorescein-NH-(CH 2 ) 2 -Gly-Arg-Val-Tyr-Ile-His-Pro-Ile-OH
[0192] Peptide analogs of Ang II were synthesized starting from 0.1 mmol Fmoc-Ile-Wang resin on an Applied Biosystems 433A peptide synthesizer. An excess of 1 mmol of preactivated amino acid (O-benzotriazol-1-yl-N,N,N',N'-tetramethyluronium hexafluorophosphate ( HBTU)). The N-terminus was bromoacetylated with 0.5 mmol bromoacetic anhydride in DMF for 30 minutes. The bromoacetylated resin was then treated with a solution of 0.5 mmol N-Boc-ethylenediamine dissolved in N-methylpyrrolidone (NMP) for 30 minutes. Peptides were cleaved from the resin with simultaneous removal of side chain protecting groups in 5 mL of TFA containing 2.5% triisopropylsilane (TIS) and 2.5% water for two hours. TFA was removed in vacuo, ether was added to the residue and the precipitated peptide was washed with ether and air dried.
[0193] 23 mg of crude peptide, 16 m...
Embodiment 2
[0195] Example 2 Tape for PET Imaging 18 Ang II analogue of F
[0196] 18 F-(CH 2 ) 3 -S-CH 2 CO-NH-(CH 2 ) 2 -Gly-Arg-Val-Tyr-Ile-His-Pro-Ile-OH
[0197]ATII peptide analogs were synthesized starting from 0.1 mmol Fmoc-Ile-Wang resin on an Applied Biosystems 433A peptide synthesizer. An excess of 1 mmol of preactivated amino acid (using HBTU) was used in the coupling step ending at arginine. The N-terminus was bromoacetylated with 0.5 mmol bromoacetic anhydride in DMF for 30 minutes. The bromoacetylated resin was then treated with a solution of 0.5 mmol N-Boc-ethylenediamine dissolved in NMP for 30 minutes.
[0198] Peptides were cleaved from the resin with simultaneous removal of side chain protecting groups in 10 mL of TFA containing 2.5% TIS and 2.5% water for two hours. TFA was removed in vacuo, ether was added to the residue and the precipitated peptide was washed with ether and air dried.
[0199] The resulting crude peptide was treated with one equivalent of...
Embodiment 3
[0200] Example 3 99m Tc-labeled Ang II analog
[0201] cPn216-Gly-Arg-Val-Tyr-Ile-His-Pro-Ile-OH
[0202]
[0203] Ang II peptide analogs were synthesized starting from 0.1 mmol Fmoc-Ile-Wang resin on an Applied Biosystems 433A peptide synthesizer. An excess of 1 mmol of preactivated amino acid (using HBTU) was used in the coupling step ending at arginine. The N-terminus was bromoacetylated with 0.5 mmol bromoacetic anhydride in DMF for 30 minutes. The resulting bromoacetyl resin was then treated with a solution of 0.2 mmol cPn216 and 0.4 NMM dissolved in DMF for 16 hours.
[0204] Peptides were cleaved from the resin with simultaneous removal of side chain protecting groups in 5 mL of TFA containing 5% TIS, 5% water and 2.5% phenol for two hours. TFA was removed in vacuo, ether was added to the residue and the precipitated product was washed with ether and air dried.
[0205] With preparative RP-HPLC (0-30% B within 40 minutes, wherein A=H 2 O / 0.1% TFA and B=CH 3 CN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com