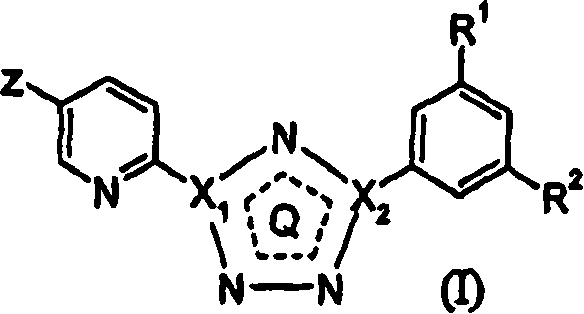
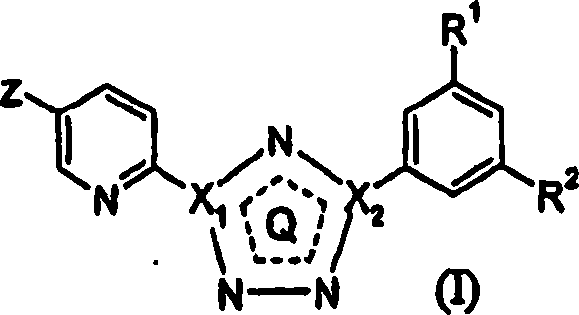
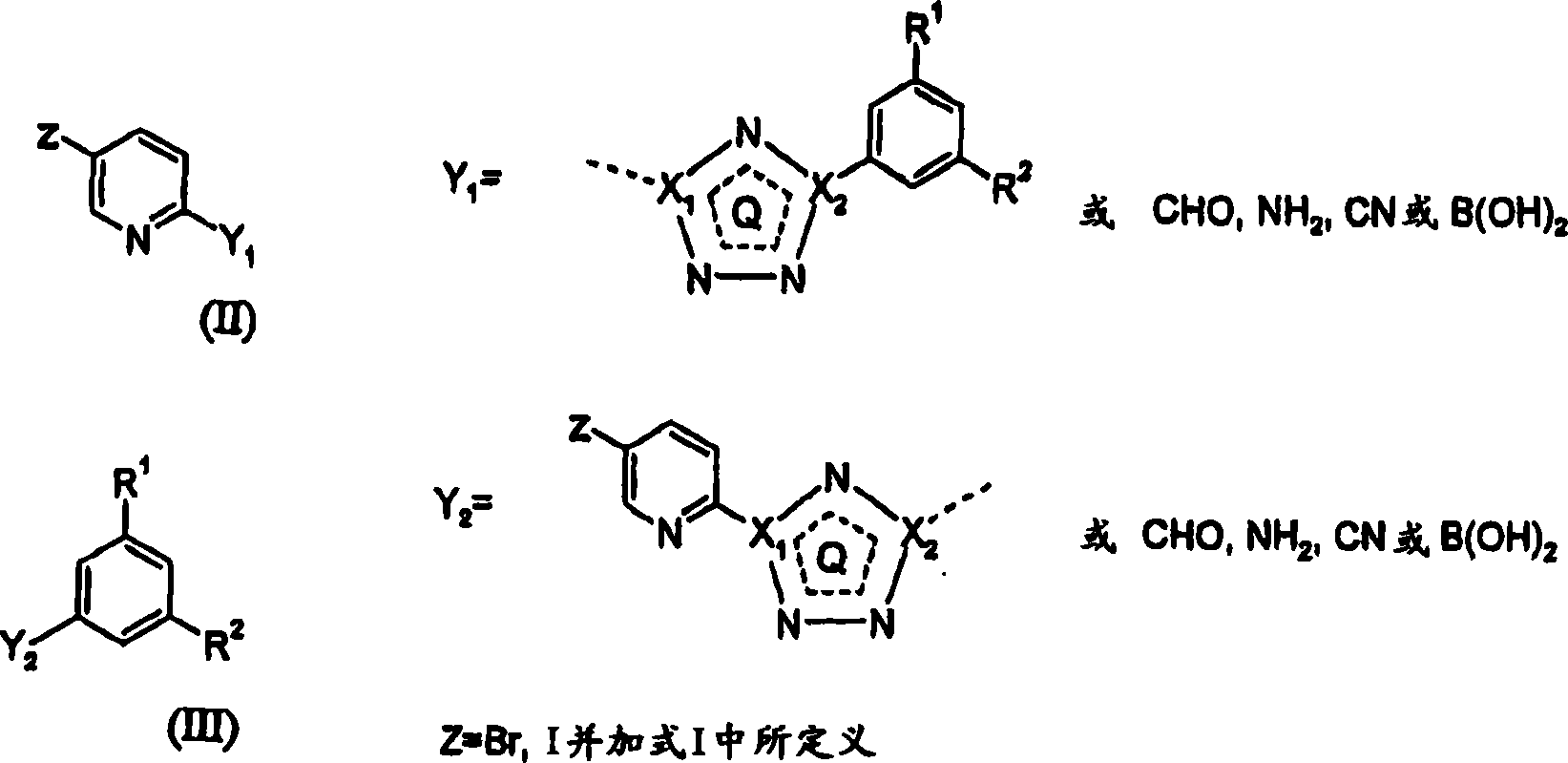
5-fluoro-, chloro- and cyano-pyridin-2-yl-tetrazoles as ligands of the metabotropic glutamate receptor-5
The technology of a compound, flupyridine, is applied in the field of new compounds, which can solve problems such as increasing the release of neurotransmitters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: 3-Fluoro-5-[5-(5-fluoropyridin-2-yl)-2H-tetrazol-2-yl] benzonitrile
[0126] The chlorination was prepared from sodium nitrite (0.06g) in ethanol (2mL), water (1mL) and 3-amino-5-fluorobenzonitrile (0.10g) in 10% aqueous HCl (2mL) at 5°C Diazonium. This solution was added dropwise to N′-[(1E)-(5-fluoropyridin-2-yl)methylene]-4-methylbenzenesulfonyl hydrazide (0.15g) in pyridine ( 5mL) in the solution so that the temperature is kept below 5°C. The reaction mixture was stirred for 2 hours, then concentrated, and the resulting product was dissolved in CH 2 Cl 2 Partition between (100 mL) and water (50 mL). The organic phase was washed with water (50mL) and brine (50mL), then dried (Na 2 SO 4 ), filtered and concentrated. The residue was recrystallized from EtOAc to obtain 40 mg of the title compound as a yellow solid.
[0127] 1 H NMR: 7.56 (m, 1H), 7.67 (m, 1H), 8.33 (m, 1H), 8.42 (m, 1H), 8.47 (m, 1H), 8.73 (m, 1H).
[0128] 3-amino-5-fluorobenzonitrile
[0129] T...
Embodiment 2
[0138] Example 2: 3-[5-(5-chloropyridin-2-yl)-2H-tetrazol-2-yl]-5-fluorobenzonitrile
[0139] Similar to 3-fluoro-5-[5-(5-fluoropyridin-2-yl)-2H-tetrazol-2-yl] benzonitrile, from 3-amino-5-fluorobenzonitrile (0.14g) And N'-[(1E)-(5-chloropyridin-2-yl)methylene]-4-methylbenzenesulfonyl hydrazide (0.22 g) to prepare the title compound to obtain 67 mg of the title compound as an orange solid.
[0140] 1 H NMR: 7.56 (m, 1H), 7.95 (m, 1H), 8.31-8.37 (2H), 8.47 (m, 1H), 8.83 (m, 1H).
[0141] N′-[(1E)-(5-chloropyridin-2-yl)methylene]-4-methylbenzenesulfonyl hydrazide
[0142] Similar to N′-[(1E)-(5-fluoropyridin-2-yl)methylene]-4-methylbenzenesulfonyl hydrazide, from 5-chloropyridine-2-carbaldehyde [J.Med.Chem. 1970, 1124-30] (1.0 g) and p-toluenesulfonyl hydrazide (1.0 g) prepared the title compound to obtain 0.54 g of the title compound as a white solid.
[0143] 1H NMR: 2.44 (s, 3H), 7.34 (m, 2H), 7.69 (m, 1H), 7.80 (d, 1H), 7.86-7.91 (3H), 8.16 (br s, 1H), 8.51 (m, 1H).
Embodiment 3
[0144] Example 3: 6-[2-(3-cyano-5-fluorophenyl)-2H-tetrazol-5-yl] nicotinonitrile
[0145] Dissolve 3-[5-(5-bromopyridin-2-yl)-2H-tetrazol-2-yl]-5-fluorobenzonitrile (WO03 / 029210) (0.03g) in DMF (3mL), And bubbling argon for 15 minutes. Add zinc cyanide (0.01g) and Pd (PPh 3 ) 4 (0.03g), the reaction was stirred at 80°C for 16 hours. CH for reaction mixture 2 Cl 2 (20mL) Dilute and use NH 4 Cl (10mL) and brine (10mL) washed, then dried (Na 2 SO4), filtered and concentrated to obtain a yellow solid, which was purified by recrystallization from EtOAc:hexane to obtain 7 mg of the title compound as a white solid.
[0146] 1 H NMR: 7.59 (m, 1H), 8.25 (m, 1H), 8.34 (m, 1H), 8.47 (m, 1H), 8.53 (m, 1H), 9.13 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com