'1,2,4' oxadiazoles as modulators of metabotropic glutamate receptor-5
A 5-membered ring, SO2 technology, used in anti-inflammatory agents, anti-toxic agents, non-central analgesics, etc., can solve problems such as increased release of neurotransmitters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1092] Preparation of compound of formula IX
[1093]
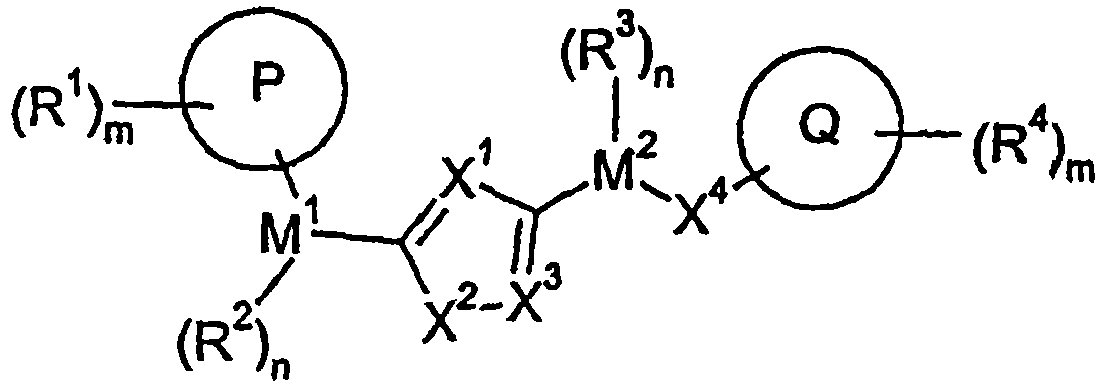
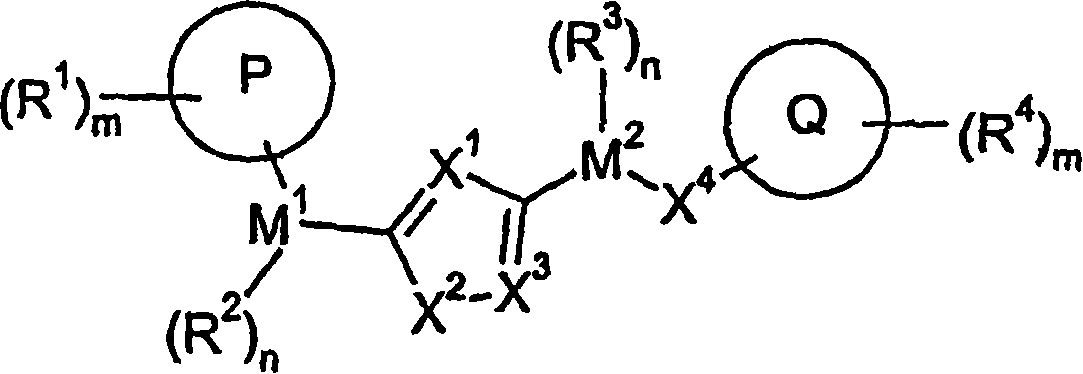
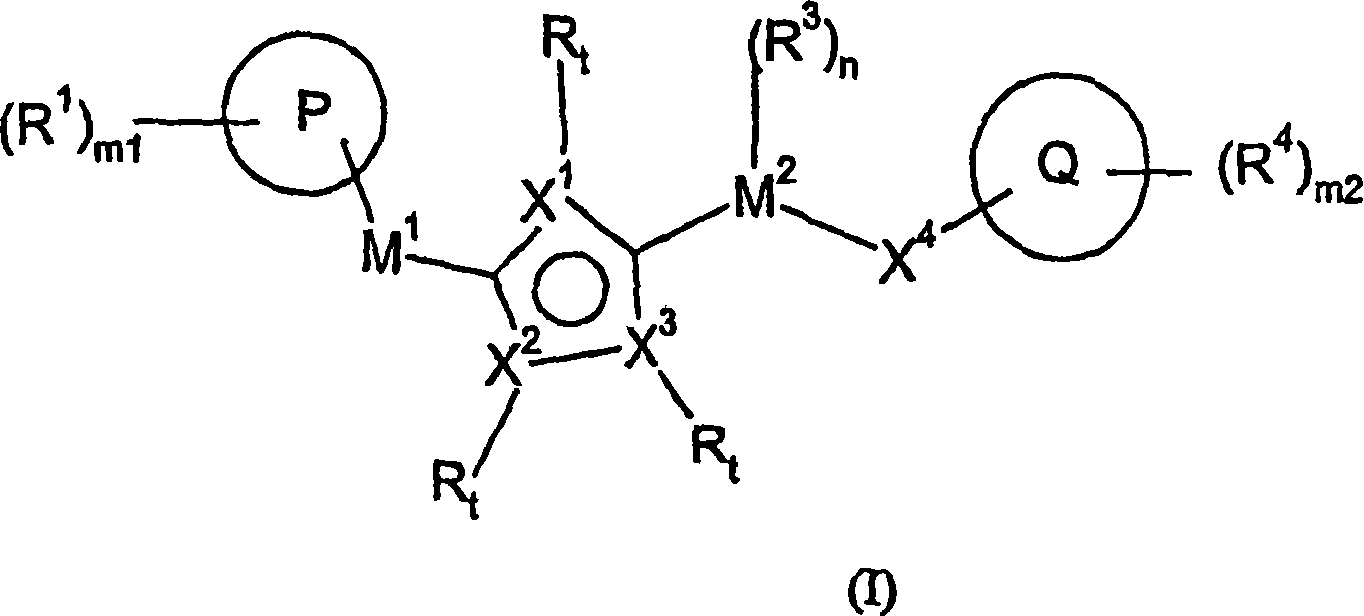
[1094] Under alkaline conditions, using a suitable base such as sodium bicarbonate or triethylamine, at a suitable temperature (0°C-100°C), in a solvent such as toluene, pass between the compound of formula VI and the compound of formula VII. , 3-dipolar cycloaddition reaction can prepare the compound of formula IX, where R 7 Independently selected from M 1 -(R 2 ) n -P-(R 1 ) m1 , M 2 -(R 3 ) n -X 4 -Q-(R 4 ) m2 And M 2 -(R 3 ) n -G, where G is a leaving group or a group that can be subsequently converted into a leaving group. The synthesis of the compound of formula VI has previously been described in the literature, for example Kim, Jae Nyoung; Ryu, Eung K; J. Org. Chem. (1992), 57, 6649-50. Using the substituted nitromethane of formula VIII, in the presence of a base such as triethylamine, at high temperature (50-100° C.), through activation with an electrophile such as PhNCO, it is also possible to achieve 1 with the d...
Embodiment 1
[1175] 6-methylpyridine-4-carboxylic acid
[1176] Put a spherical flask filled with hydrogen with 2-chloro-6-methylpyridine-4-carboxylic acid (2g, 12.0mmol), 10% (weight) palladium activated carbon (0.5g), and triethylamine (4.8ml) Connect to a flask of ethanol (24 ml), and then stir overnight at room temperature. The reaction mixture was filtered through Celite, washed with methanol and concentrated. The residue was triturated with dichloromethane and then filtered to give 6-picoline-4-carboxylic acid (white solid); 1.05 g (66%). 1 H NMR (MeOD) δ (ppm): 8.62 (d, 1H), 7.68 (s, 1H), 7.60 (d, 1H), 2.55 (s, 3H).
Embodiment 2
[1178] 1-cyano-3-ethylbenzene
[1179] Blow argon into a solution of 1-bromo-3-ethylbenzene (2.5g, 13.5mmol) in DMF (37ml) for 10 minutes, then add zinc cyanide (1.75g, 14.9mmol) and tetrakis(triphenyl) Phosphine) Palladium(O) (1.56 g, 1.35 mmol). After stirring overnight at 80°C, the reaction mixture was diluted with ethyl acetate (35 ml) and then filtered through Celite to remove the precipitate. The filtrate was washed with water (3x), saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated. The obtained product was purified by flash column chromatography (using 2% ethyl acetate / hexane) to obtain a colorless liquid (1.42 g). GC-MS (M+): 131.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com