Dichloroacetate combined with inotrope for heart protection
A dichloroacetate, heart protection technology, applied in the field of dichloroacetate, can solve the problems of increasing myocardial oxygen consumption, not improving mechanical efficiency, and increasing cardiac muscle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0068] Methods used in the studies described in Examples A to C
[0069] The studies described in Examples A to C describe three different clinical studies of the effects of DCA when administered to patients during and / or after cardiac surgery.
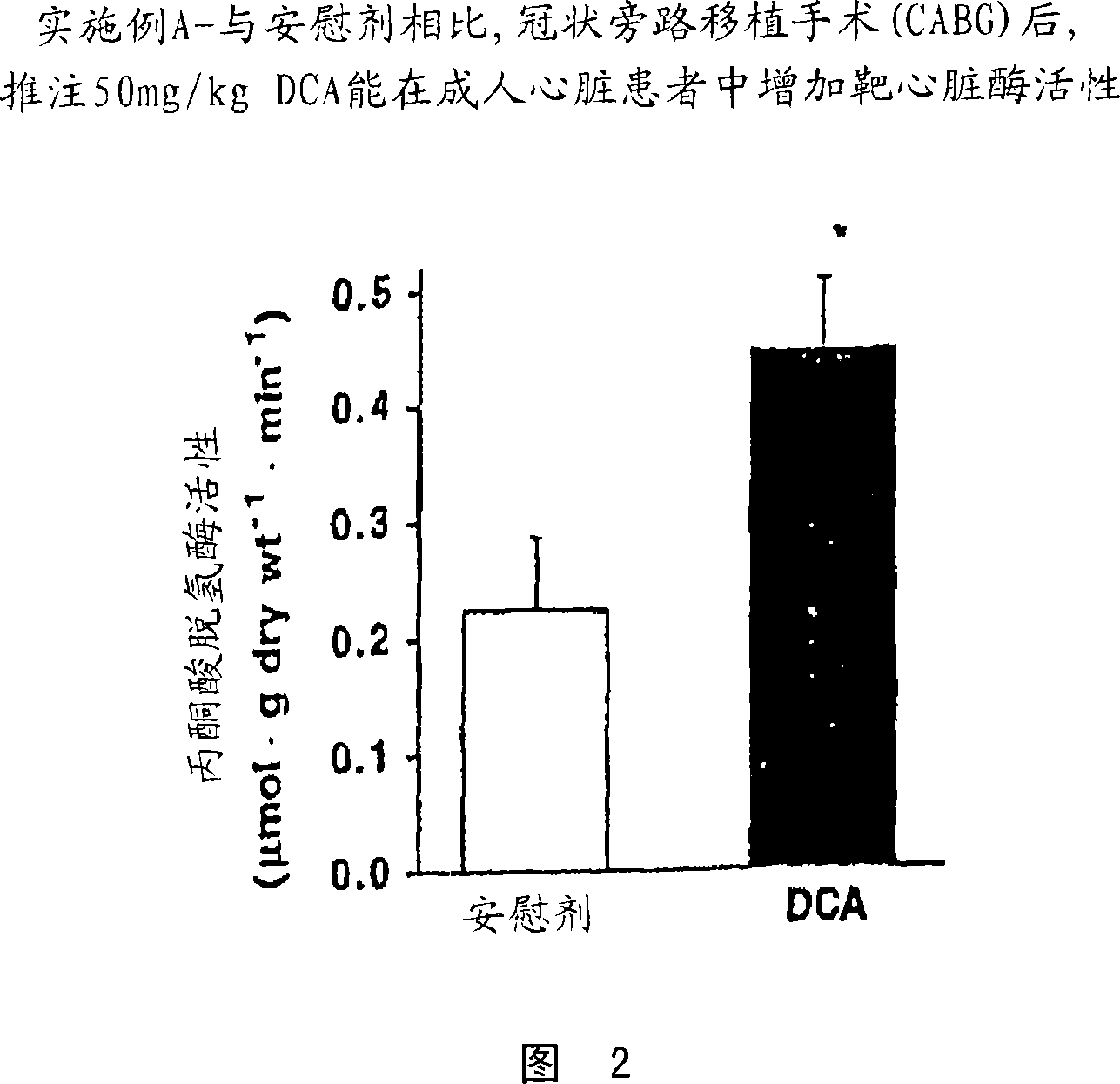
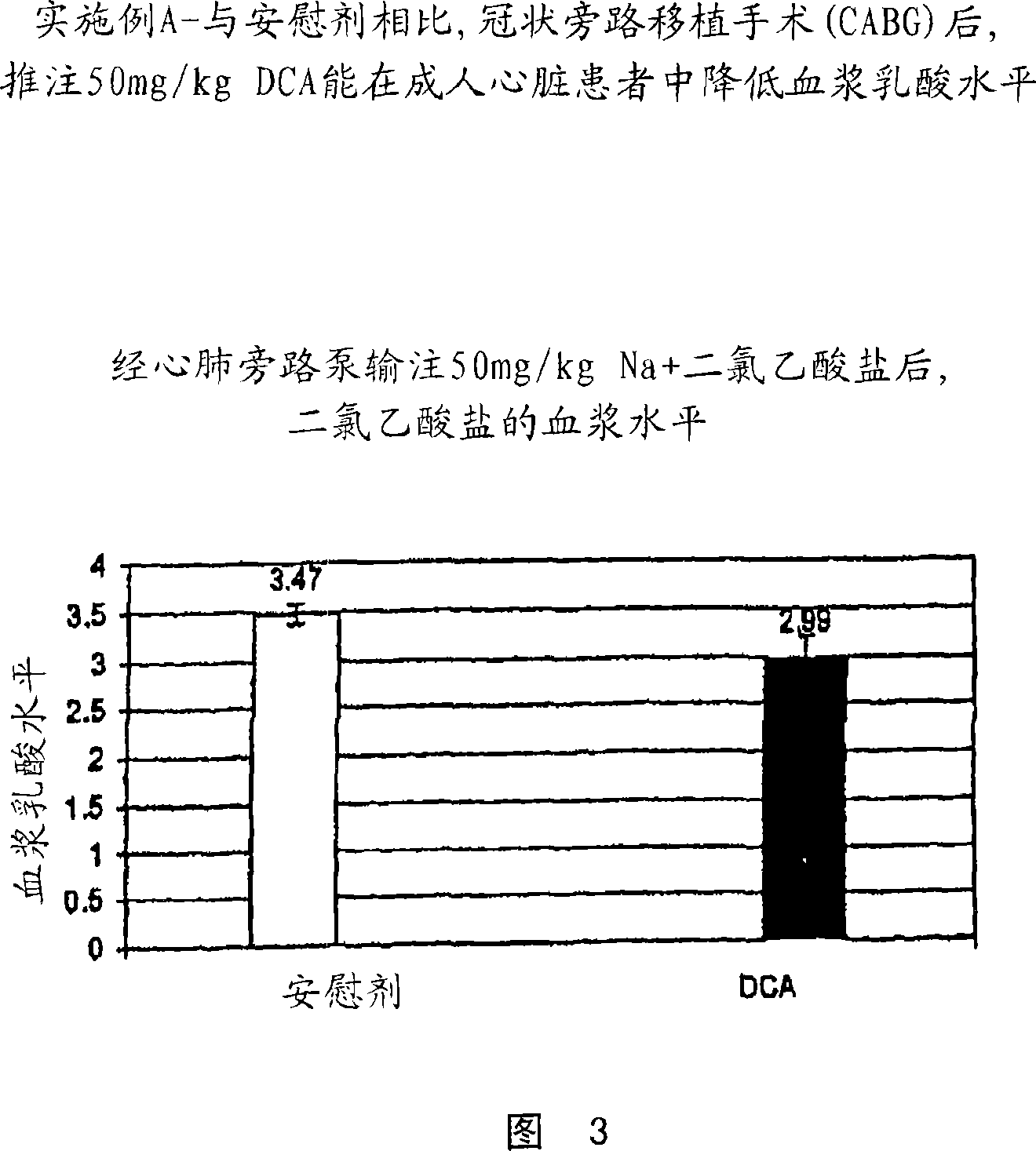
[0070] The study described in Example A involved adult patients investigating the effects of DCA on cardiometabolism. DCA is administered to patients undergoing elective cardiac bypass grafting (CABG). The study was conducted in coronary artery bypass grafting in the presence of clinically recommended doses of hemodynamic drugs.
[0071] The study described in Example B involved the administration of a single bolus dose of DCA to pediatric patients undergoing cardiac surgery to correct congenital heart defects. This regimen was performed in the presence of clinically recommended hemodynamic agents, and it was determined that the dose and total amount of these agents would be reduced when combined with DCA.
[0072] The study de...
Embodiment A
[0074] Description of the research protocol
[0075] DCA or saline was administered to 18 patients undergoing elective cardiac bypass grafting (CABG), with DCA (50 mg / kg in 100 ml saline) injected into the aortic root in a double-blind randomized fashion just before removal of the aortic cruciform or placebo. Based on the pharmacokinetics of DCA, we would expect this to yield plasma concentrations of approximately 1 mM. The study consisted of 8 patients treated with DCA and 10 patients treated with placebo.
[0076] 1. Intervention
[0077] a. "Conventional" treatment
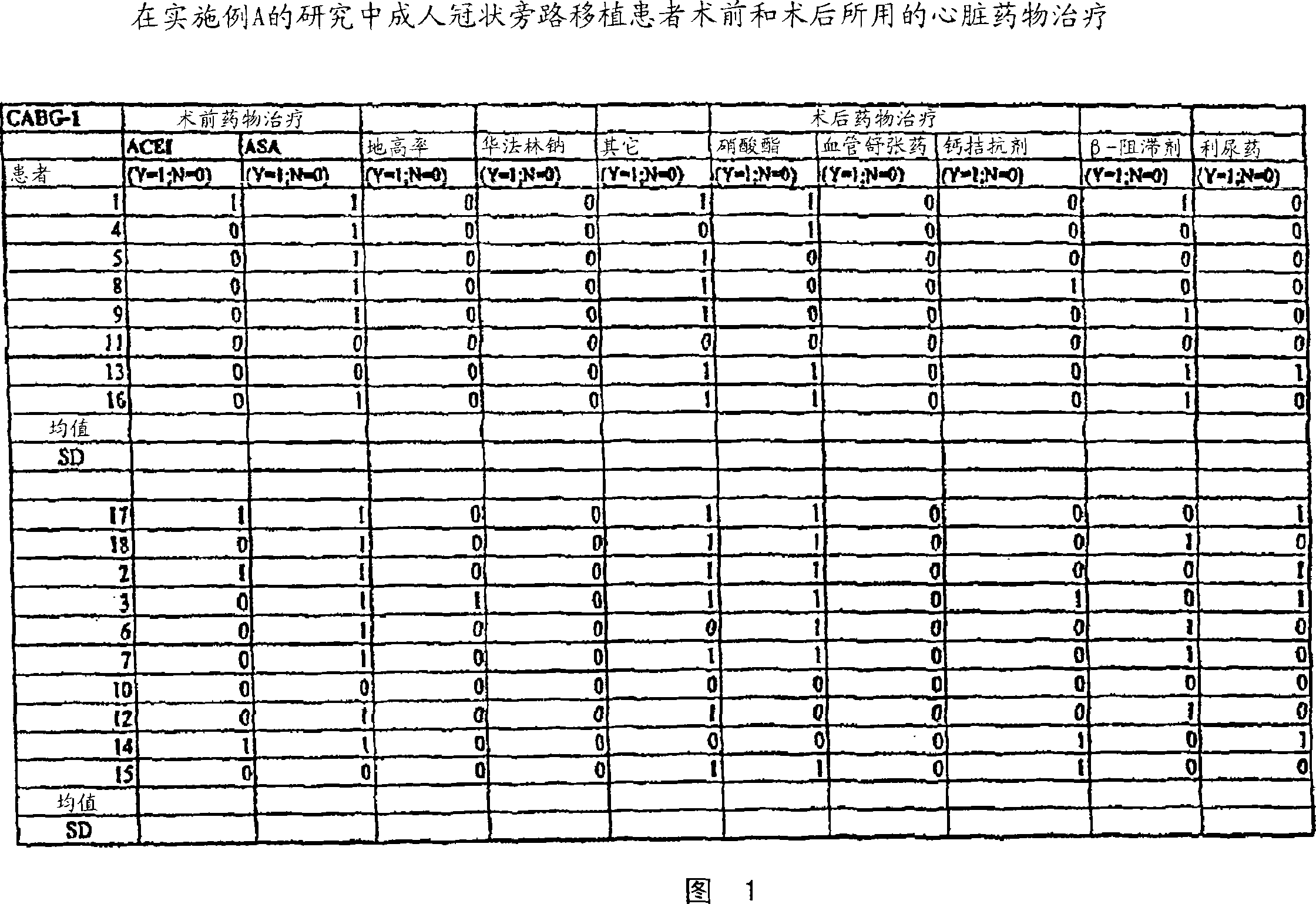
[0078] All procedures and medications for patients normally administered CABG are routinely applied. A list of medications provided to these patients is shown in Figure 1.
[0079] b. "Intervention" therapy
[0080] The intervention involved intra-aortic root injection of DCA (50 mg / kg) or placebo just prior to removal of the aortic cross clip. The coded solution was formulated such that a dose of 1 ml / k...
Embodiment B
[0092] Description of the research protocol
[0093] This study was a randomized, placebo-controlled, double-blind, one-surgeon study of the use of DCA in 40 high-risk pediatric patients requiring cardiac surgery to correct a complex congenital heart defect.
[0094] 1. Research groups
[0095] In this trial, 40 children were recruited to participate in a surgeon's study, of which 18 received DCA and 22 received placebo. The 1995 power calculation is based on the separation of the CPB-1 trial of n=40 patients.
[0096] 2. Criteria for included studies
[0097] a. The age is less than 1 year old.
[0098] b. Parental or guardian consent.
[0099] c. Need for open heart surgery to correct complex congenital heart defects (eg, Tetralogy of Fallot).
[0100] d. Surgeons, anaesthetists and cardiologists agree.
[0101] e. There are no serious cardiac complications that would prevent the implementation of the study protocol.
[0102] 3. Exclusion criteria
[0103] a. Lack of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com