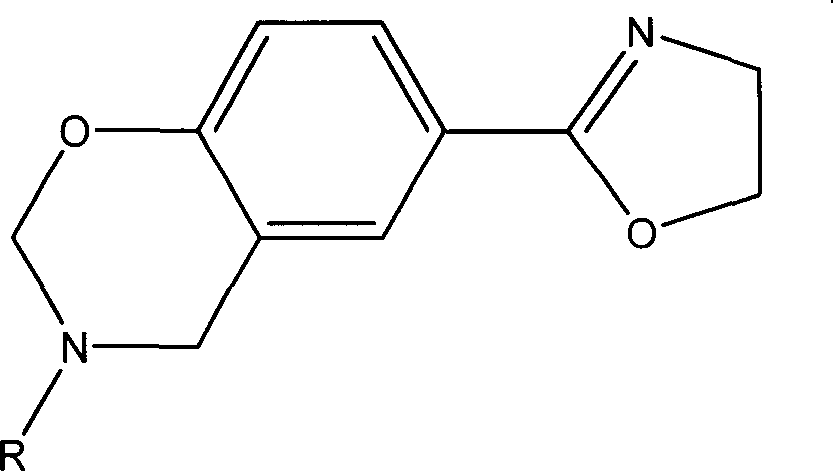
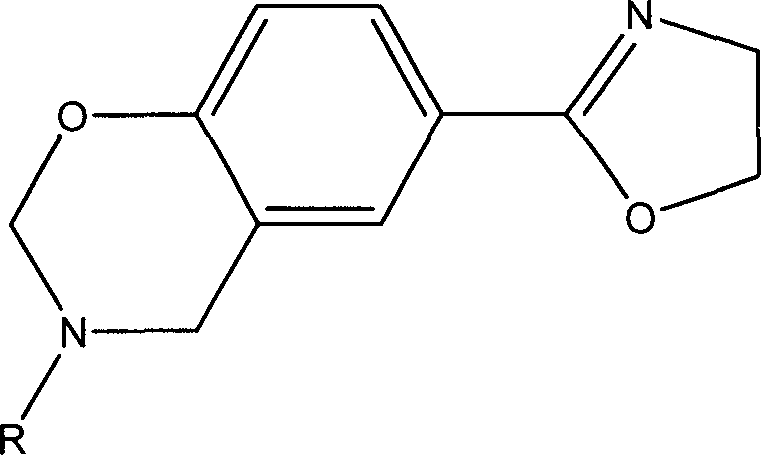
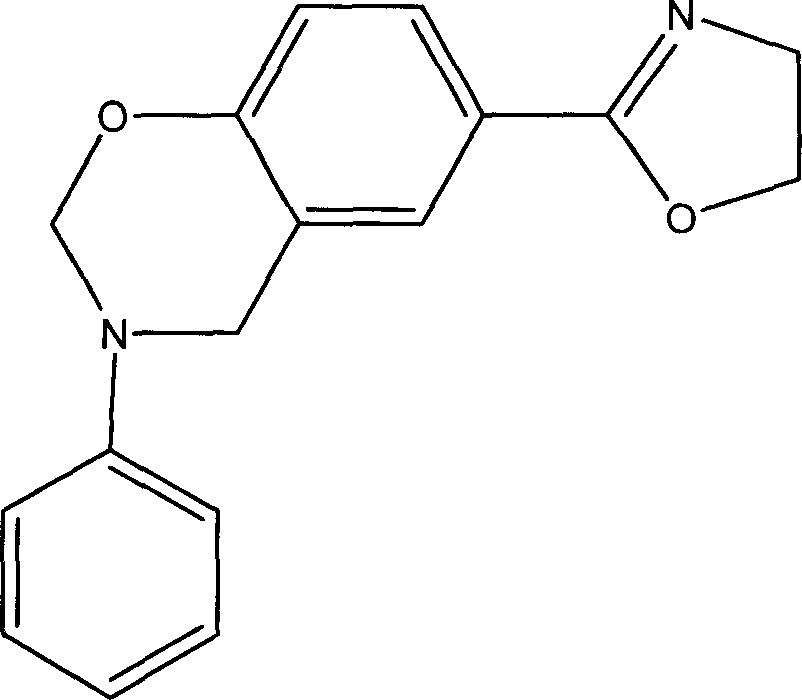
2-oxazolinyl-benzo oxazinyl compound and its composition and preparing method
A technology of benzoxazine and oxazoline, which is applied in the field of 2-oxazoline-benzoxazine compounds and their compositions and preparations, can solve the problem of 2-oxazoline-benzoxazine which has not been seen yet. To solve the problems such as the report of intermediates, to achieve the effect of improving flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Adopt the method of solution, take triazine as reaction raw material, prepare p-(2-oxazoline)-3-phenyl-3,4-dihydro-2H-1,3-benzoxazine, abbreviated as POB, Its structure is as follows:
[0031]
[0032] Dissolve 2-(p-hydroxyphenyl)-2-oxazoline in DMF at room temperature, and add 1,3,5-triphenyl-hexahydro-1,3 in a molar ratio of 3:1:3, 5-Triazine and paraformaldehyde. After stirring evenly, the temperature was raised to 90°C. After reacting for 2 hours, part of DMF was distilled off under reduced pressure, cooled and left standing to obtain a light yellow translucent viscous body. Add an appropriate amount of deionized water to the obtained product at room temperature, add an appropriate amount of ether after stirring, and let it stand after stirring evenly. After stabilization, take out the light yellow transparent solution in the upper layer. After washing with alkali and water, distill out the ether under reduced pressure, and the obtained The product was dried in...
Embodiment 2
[0035] Prepare p-(2-oxazoline)-3-allyl-3,4-dihydro-2H-1,3-benzoxazine by solution method, abbreviated as POAB, its structure is as follows:
[0036]
[0037] 2-(p-Hydroxyphenyl)-2-oxazoline was dissolved in DMF at room temperature, and allylamine and paraformaldehyde were added sequentially at a molar ratio of 1:1:2. After stirring evenly, the temperature was raised to 90°C. After reacting for 2 hours, part of DMF was distilled off under reduced pressure, cooled and left standing to obtain a light yellow translucent viscous body. Add an appropriate amount of deionized water to the obtained product at room temperature, add an appropriate amount of ether after stirring, and let it stand after stirring evenly. After stabilization, take out the light yellow transparent solution in the upper layer. After washing with alkali and water, distill out the ether under reduced pressure, and the obtained The product was dried in a vacuum oven at 45°C for 24 hours under vacuum to obtain ...
Embodiment 3
[0039] Prepare p-(2-oxazoline)-3-furyl-3,4-dihydro-2H-1,3-benzoxazine by solution method, abbreviated as POFB, its structure is as follows:
[0040]
[0041] The method is the same as in Example 2, except that allylamine is replaced by furylamine, and finally a light yellow viscous body is obtained with a yield of 69.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com