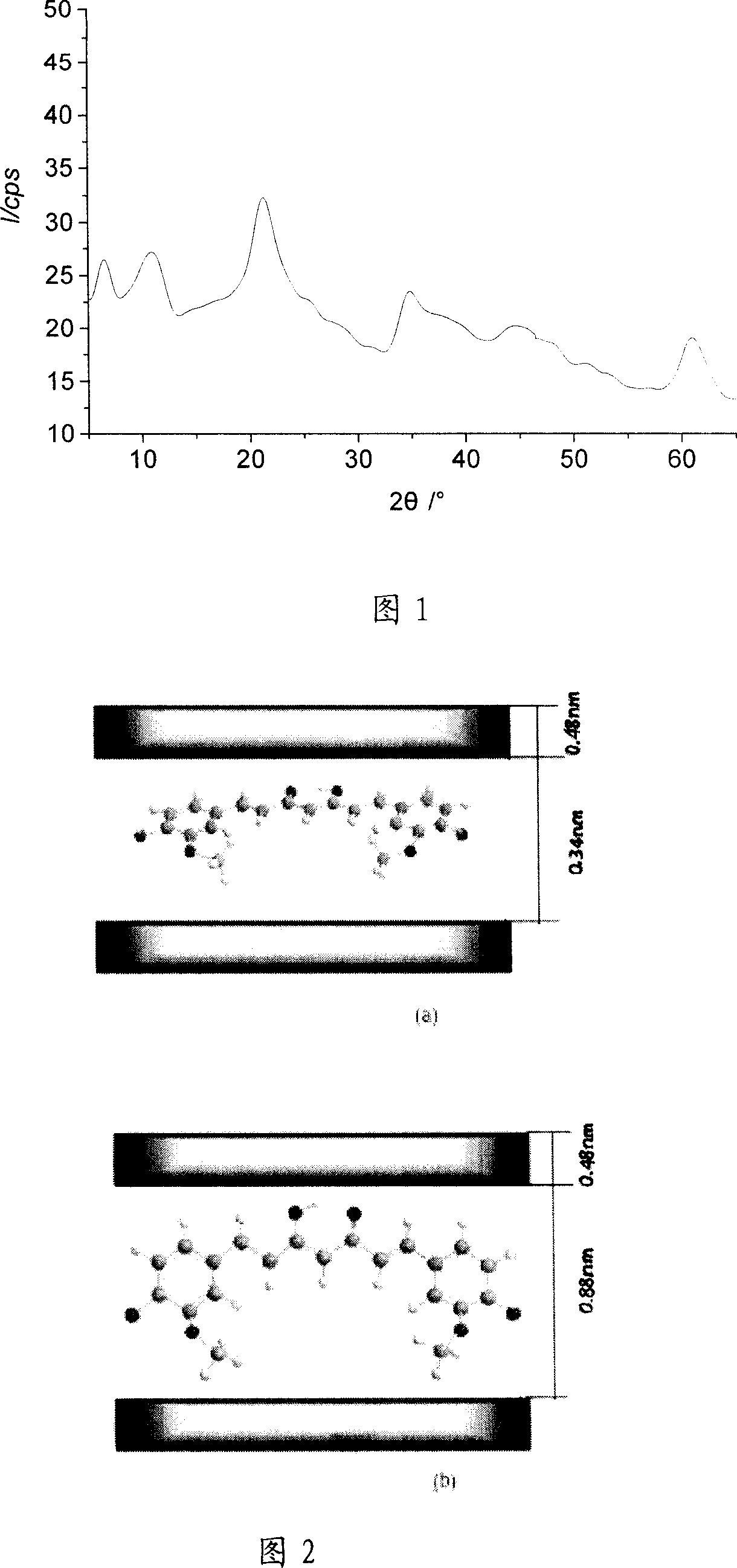
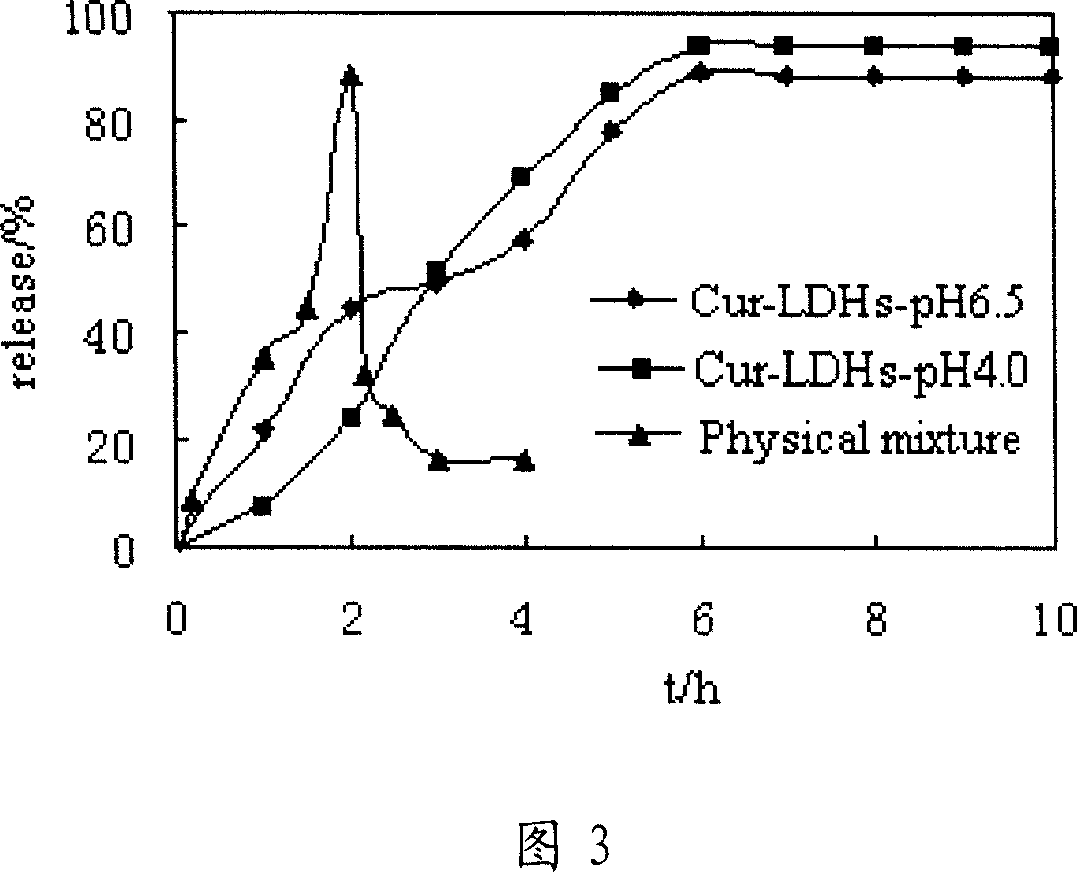
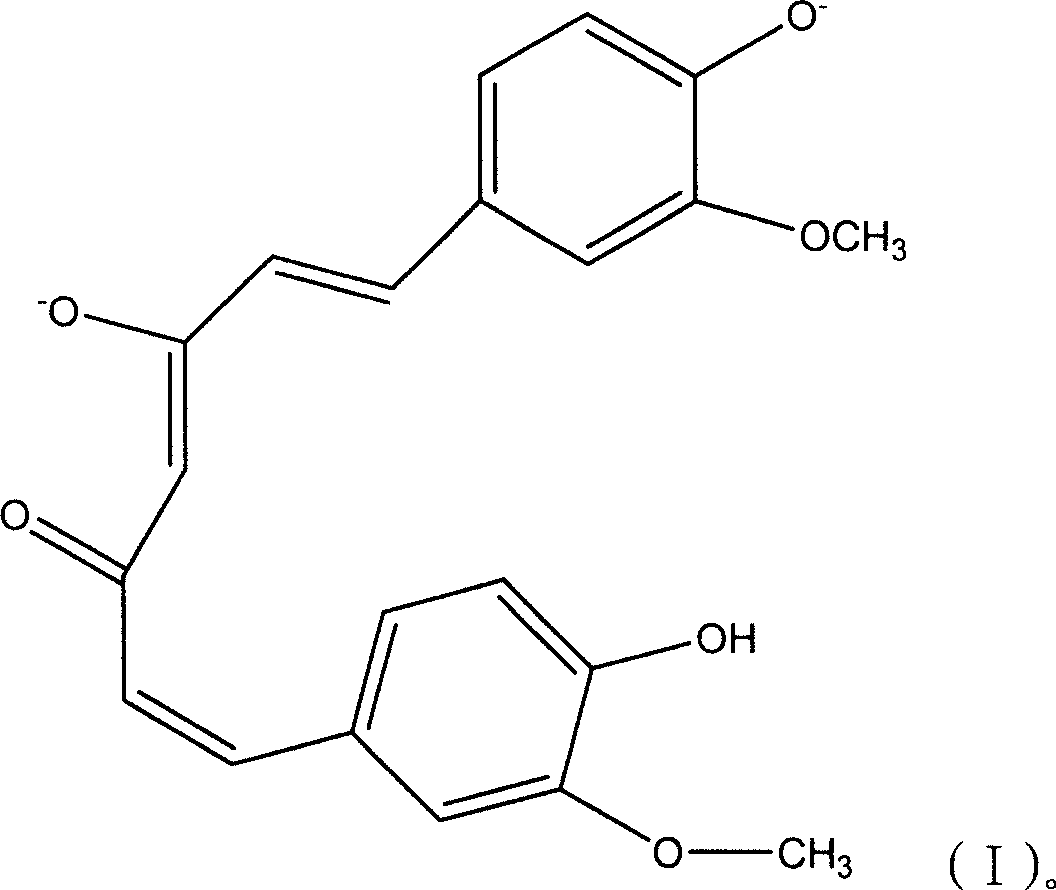
Sustained release agent of curcumin and preparation method thereof
A technology of curcumin and sustained-release agent, which is applied in the field of curcumin sustained-release agent and its preparation, can solve the problems of low bioavailability of curcumin, achieve the effect of improving utilization and improving drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Get 1.034g (3mmol) curcumin (full name curcumin, be called for short Cur) dissolve in 30mL deionized water, adjust the pH value to be 10 with 2mol / L NaOH solution, obtain solution B; Take 1.53g (6mmol) Mg(NO 3 ) 2 ·6H 2 O and 0.75g (2mmol) Al(NO 3 ) 3 9H 2 O was dissolved in 10 mL of deionized water, and stirred to dissolve to obtain solution A. Under vigorous stirring, drop solution A into solution B, N 2 Protection, reaction at room temperature for 48h. The product was filtered, washed and dried in an oven. The deionized water used in the process is all deionized water with carbon dioxide removed.
[0050] The chemical analysis result of the curcumin sustained-release agent obtained at last is: Mg:Al:Cur=1.96:1:0.42. The specific chemical composition can be derived from the relationship between x, a, and b.
Embodiment 2
[0052] a. Take 0.594g (0.002mol) of Zn(NO 3 ) 2 ·6H 2 O and 0.375g (0.001mol) of Al(NO 3 ) 3 9H 2 O was dissolved in 100ml deionized water to make solution A;
[0053] b. Dissolve 4.8g of NaOH solid in 100ml of deionized water to form solution C;
[0054] c.N 2 Under protection, 10 parts by volume of solution A was added dropwise to 1 part by volume of solution C at a certain dropping rate, and the pH of the final solution was adjusted to be between 9 and 10, and stirring was continued for half an hour after the dropwise addition. After the obtained slurry was crystallized at 65°C for 18 hours, it was suction-filtered, washed with deionized water until neutral, dried at 65°C, ground, weighed, and stored in a desiccator.
[0055] d. Dissolve 1.034g (0.003mol) Cur in 300mL deionized water to obtain solution D;
[0056] e. the product of step c is dissolved in pure water, and it is prepared into a solution E with an Al ion concentration of 0.01mol / L;
[0057] f. Mix solu...
Embodiment 3
[0061] a. Take 1.485g (0.005mol) Zn(NO 3 ) 2 ·6H 2 O and 0.375gAl(NO 3 ) 3 9H 2 O (0.001mol) was dissolved in 100ml deionized water to make solution A;
[0062] b. Dissolve 4.8g of NaOH solid in 100ml of deionized water to form solution C;
[0063] c.N 2 Under protection, 10 parts by volume of solution A was added dropwise to 1 part by volume of solution C at a certain dropping rate, and the pH value of the final solution was adjusted to be between 9 and 10, and stirring was continued for half an hour after the dropwise addition. After the obtained slurry was crystallized at 65°C for 18 hours, it was suction-filtered, washed with deionized water until neutral, dried at 65°C, ground, weighed, and stored in a desiccator.
[0064] d. Dissolve 1.034g (0.003mol) Cur in 15mL deionized water to obtain solution D;
[0065] e. the product of step c is dissolved in pure water to be prepared as a solution E with an Al ion concentration of 0.1mol / L;
[0066] f. Mix solutions D an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com