Methods of treating affective disorders using derivatives of (-)- venlafaxine
a technology of o-desmethylvenlafaxine and derivatives, which is applied in the direction of optically active compound separation, diaryl/thriaryl methane dye, diarylmethane dye, etc., can solve the problems of limiting the dose level, frequency and duration of drug therapy, and hampered studies directed at understanding the activity of o-desmethylvenlafaxine as compared to its parent, and achieves high purity and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
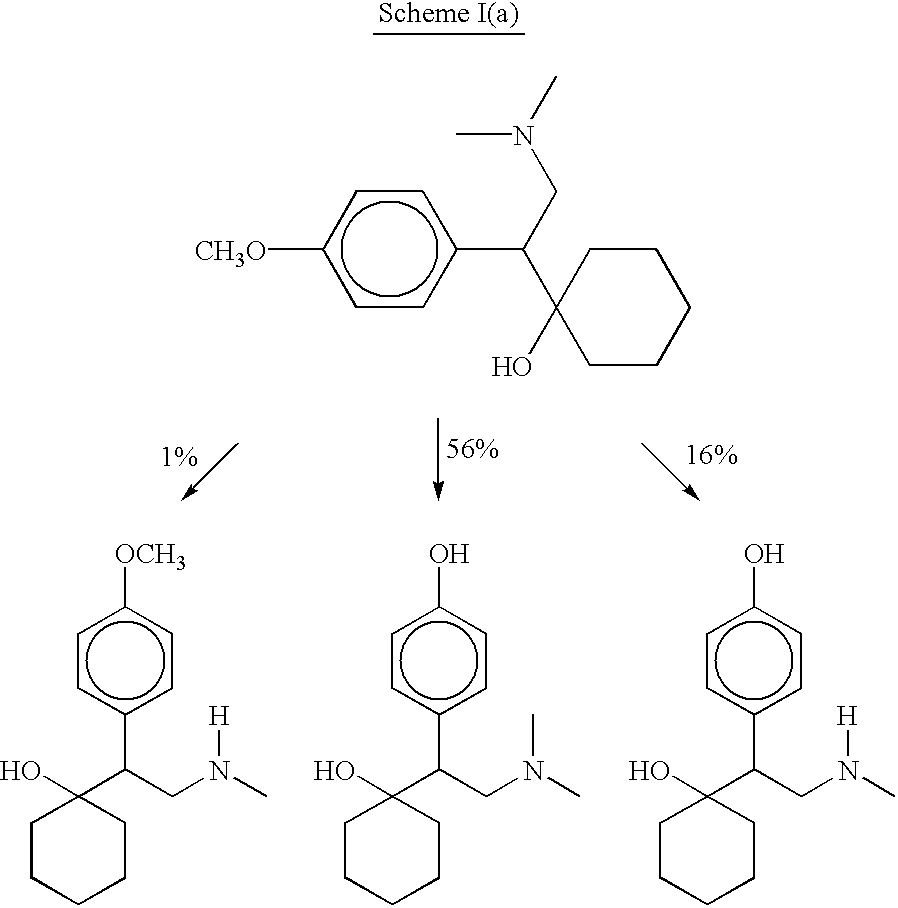
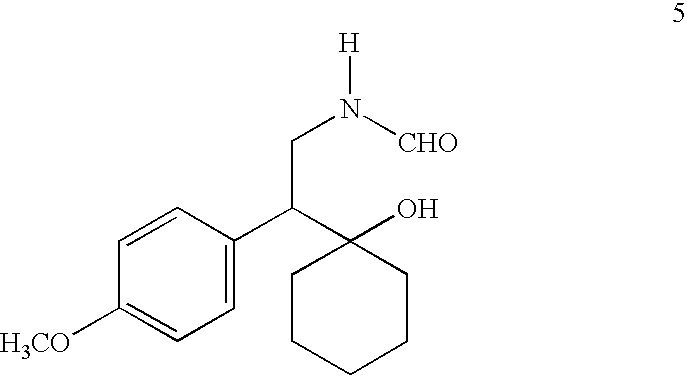
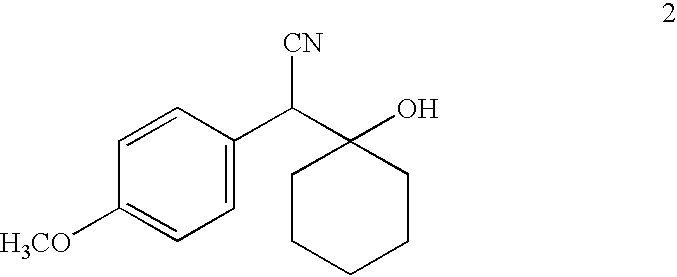
[0024] This invention relates to optically pure derivatives of (-)-venlafaxine such as, but not limited to, (-)-O-desmethylvenlafaxine, (-)-N-desmethylvenlafaxine, and (-)-N,O-didesmethylvenlafaxine. This invention further relates to the synthesis of optically pure (-)-venlafaxine derivatives and to compositions (e.g., pharmaceutical compositions) comprising them. The invention also relates to novel uses of the compounds disclosed herein, which constitute improvements over the use of racemic venlafaxine as well as over the optically pure isomers of venlafaxine.
[0025] One embodiment of the invention encompasses a method of treating an affective disorder in a human which comprises administering to a human in need of such treatment a therapeutically effective amount of a (-)-venlafaxine derivative, preferably (-)-O-desmethylvenlafaxine, or a pharmaceutically acceptable salt thereof, substantially free of its (+) stereoisomer. Venlafaxine derivatives, preferably (-)-O-desmethylvenlafax-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com