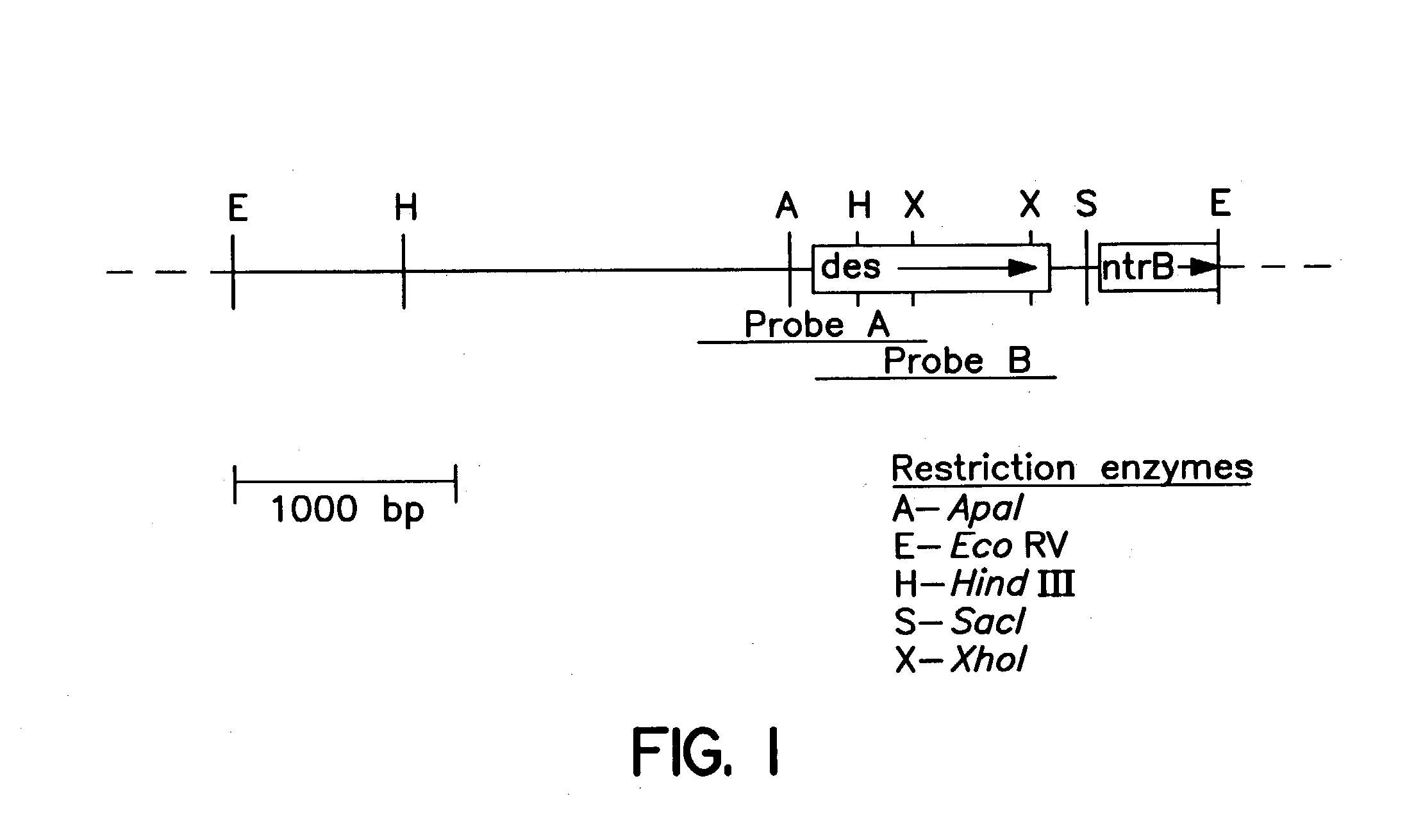
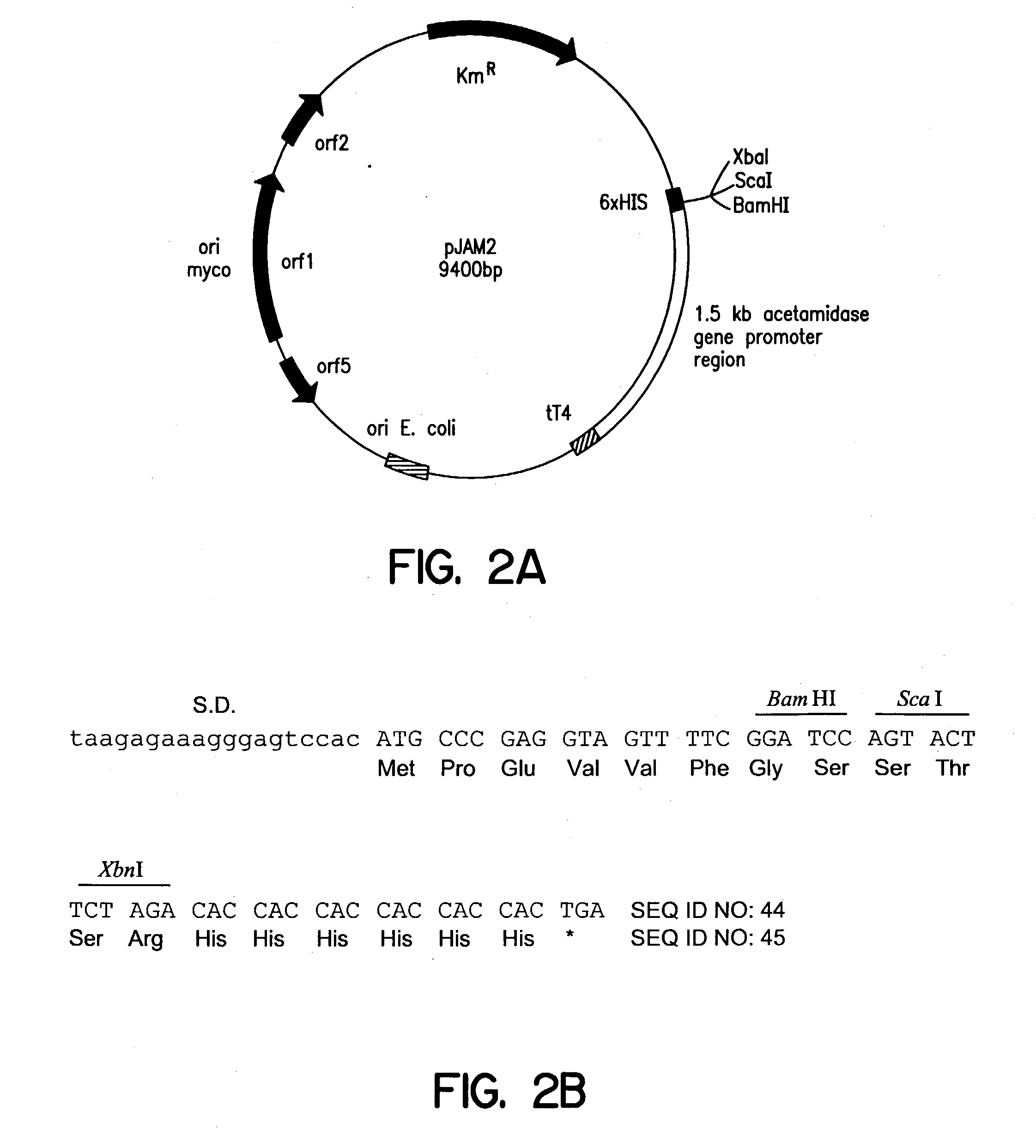
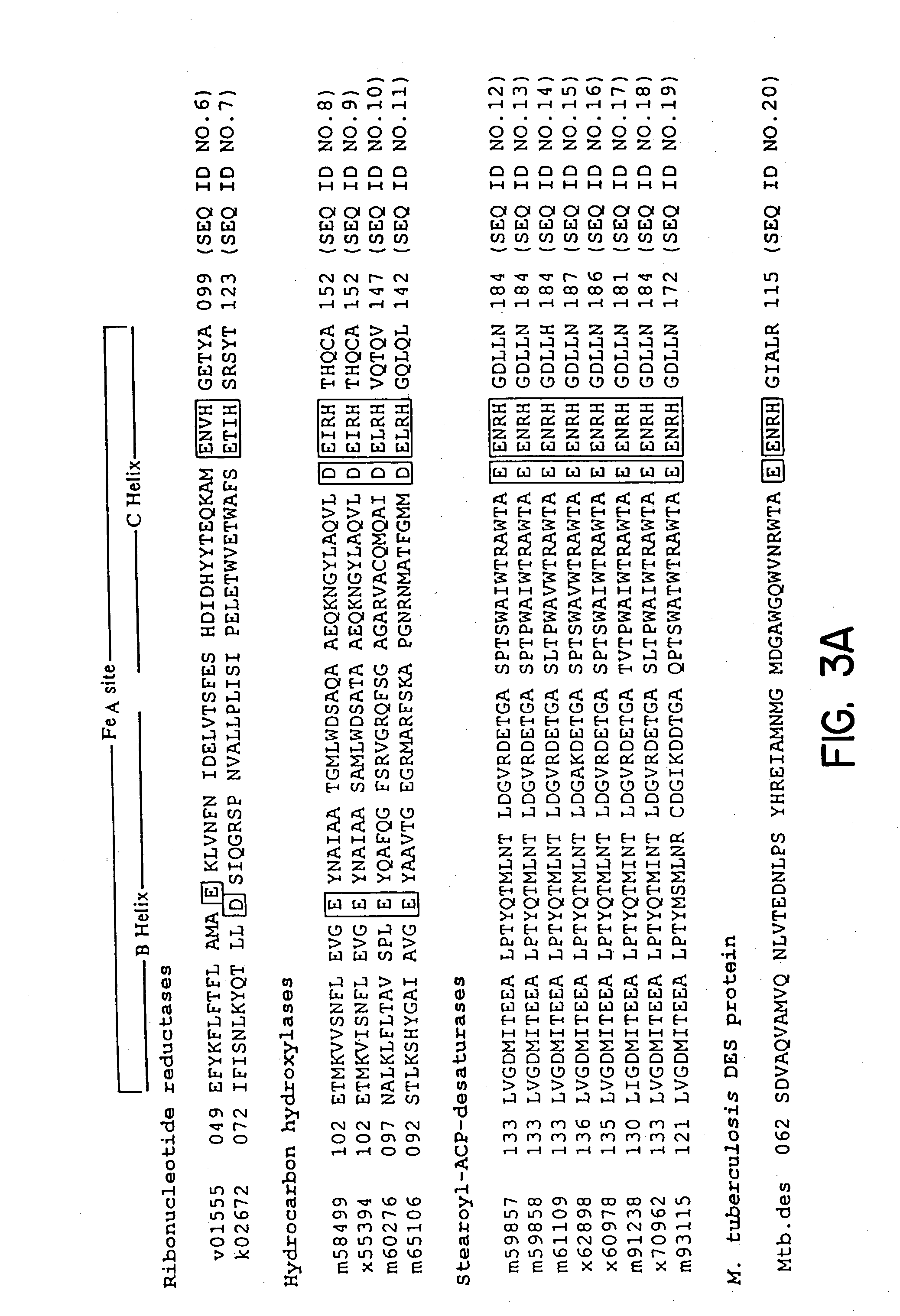
Method of screening anti-mycobacterial molecules
a technology of mycobacterial molecules and anti-mycobacterial agents, which is applied in the field of screening anti-mycobacterial molecules, can solve the problems that the typical mycobacterial infection is often difficult to cur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0049] Bacteria, Media and Growth Conditions
[0050] The bacterial strains and plasmids used in this study are listed in FIG. 8. E. coli DH5a or BL21 (DE3) pLysS cultures were routinely grown in Luria B medium (Difco) at 37.degree. C. Mycobacterium cultures were grown in Middlebrook 7H9 medium (Difco) supplemented with Tween 0.05%, glycerol (0.2%) and ADC (glucose, 0.2%; BSA fraction V, 0.5%; and NaCl, 0.085%) at 37.degree. C. When required, antibiotics were added at the following concentrations: ampicillin (100 .mu.g / ml), kanamycin (20 .mu.g / ml).
[0051] Human and Cattle Sera
[0052] Serum specimens from 20 individuals with pulmonary or extra-pulmonary tuberculosis (M. tuberculosis infected) were obtained from the Bligny sanatorium (France). Six sera from M. bovis infected human tuberculous patients and 24 sera from BCG-vaccinated patients suffering from other pathologies were respectively obtained from Institut Pasteur, (Madagascar), and the Centre de Biologie Mdicale spcialise (CBMS) (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com