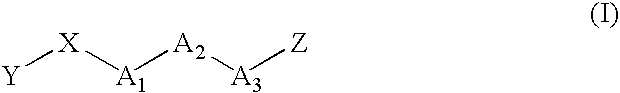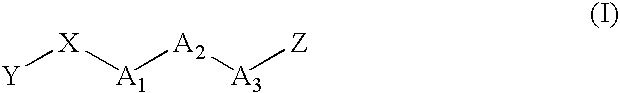Substituted heterocyclic acyl-tripeptides useful as thrombin receptor modulators
a technology of acyltripeptides and thrombin receptors, which is applied in the direction of drug compositions, peptide/protein ingredients, extracellular fluid disorders, etc., and can solve problems such as the need to adjust dosages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
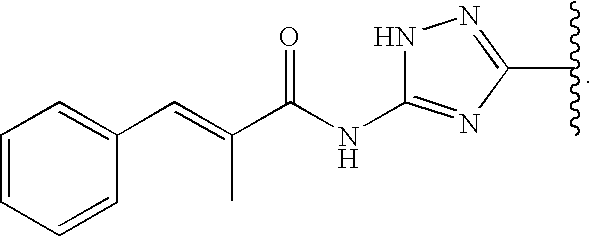
N-(5-Bromopyridin-3-yl-carbonyl)-cyclohexylalanyl-argininyl-phenylalanine Amide (2)
[0081] 11
[0082] Fmoc-phenylalanine amide (3.87 g, 10 mm) was stirred in ACN (100 mL) and DEA ( 5 mL) was added and stirred at RT for 1 hr. The solution was evaporated in vacuo to an oil, which was triturated 3.times. with hexane (100 mL) and dissolved in ACN (100 mL); Fmoc-Arg(PMC)-OH (6.63 g, 10 mm) and HOBT (1.53 g, 10 mm) were added, followed by DCC (4.1 g, 20 mm) and solution was stirred at RT. The urea by-product was filtered and the filtrate was evaporated in vacuo to an oil, which was triturated 3.times. with hexane (100 mL). The crude product was stirred in ACN (100 mL) and DEA (5 mL) was added and stirred at RT for 1 hr. The solution was evaporated in vacuo to an oil, which was triturated 3.times. with hexane (100 mL) to a solid. This dipeptide was combined in ACN (100 mL) with Fmoc-Cha-OH (3.93 g, 10 mm) and HOBT (1.53 g, 10 mm) and then DIC (2.52 g, 20 mm) was added and reaction stirred at ...
example 3
[0083] As a specific embodiment of an oral composition, 100 mg of the compound 1 of Example 1 is formulated with sufficient finely divided lactose to provide a total amount of 580 to 590 mg to fill a size O hard gel capsule.
[0084] BIOLOGY
[0085] The compounds of the present invention modulate platelet activation induced by thrombin's proteolytic cleavage of its platelet surface receptor, and thereby activate / inhibit platelet aggregation. Compounds that exhibit agonist activity may be expected to aid in wound healing and tissue repair, while antagonist compounds may be useful in treating platelet-mediated thrombotic disorders such as arterial and venous thrombosis, acute myocardial infarction, reocclusion following thrombolytic therapy and angioplasty, and a variety of vaso-occlusive disorders.
example 4
[0086] IN VITRO THROMBIN RECEPTOR BINDING ASSAY.
[0087] CHRF membranes (Jones, Biochim. Biophys. Acta 1992, 1136, 272) are thawed from -70.degree. C., centrifuged at maximum speed for 5 min, washed twice with binding buffer (50 mM HEPES containing 5 mM MgCl.sub.2 and 0.1% BSA), and re-suspended in binding buffer (25 g / 100 mL). 100 l membranes are added to the 24-Wallac plates and delivered to the Tomtech apparatus. In a typical experiment, 6 l of samples (from a 125 g / mL intermediary plate, 20% DMSO) and 44 l buffer are delivered to the plates (final conc. of compounds is 3.7 g / mL, 0.6% DMSO). Similarly, 6 l 20% DMSO and 44 l buffer are delivered to both column 1 (NSB) and column 12 (TB). 10 l Ser-pFPhe-Har-Leu-Har-Lys-Tyr-NH.sub.2 (721-40; 500 M i n deionized water) is added to column 1. 50 l tritiated 721-40 (specific activity 46 Ci / mmol) is added to all the wells. The plates are mixed well for 20 seconds, incubated for 30 min, and then harvested with 10 mM HEPES / 138 mM NaCl using ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| vascular permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com