Constrained himbacine analogs as thrombin receptor antagonists
A drug and carrier technology, used in anti-inflammatory agents, drug combinations, blood diseases, etc.
Inactive Publication Date: 2011-06-22
MERCK & CO INC
View PDF7 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Formula I
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment A
Embodiment B
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
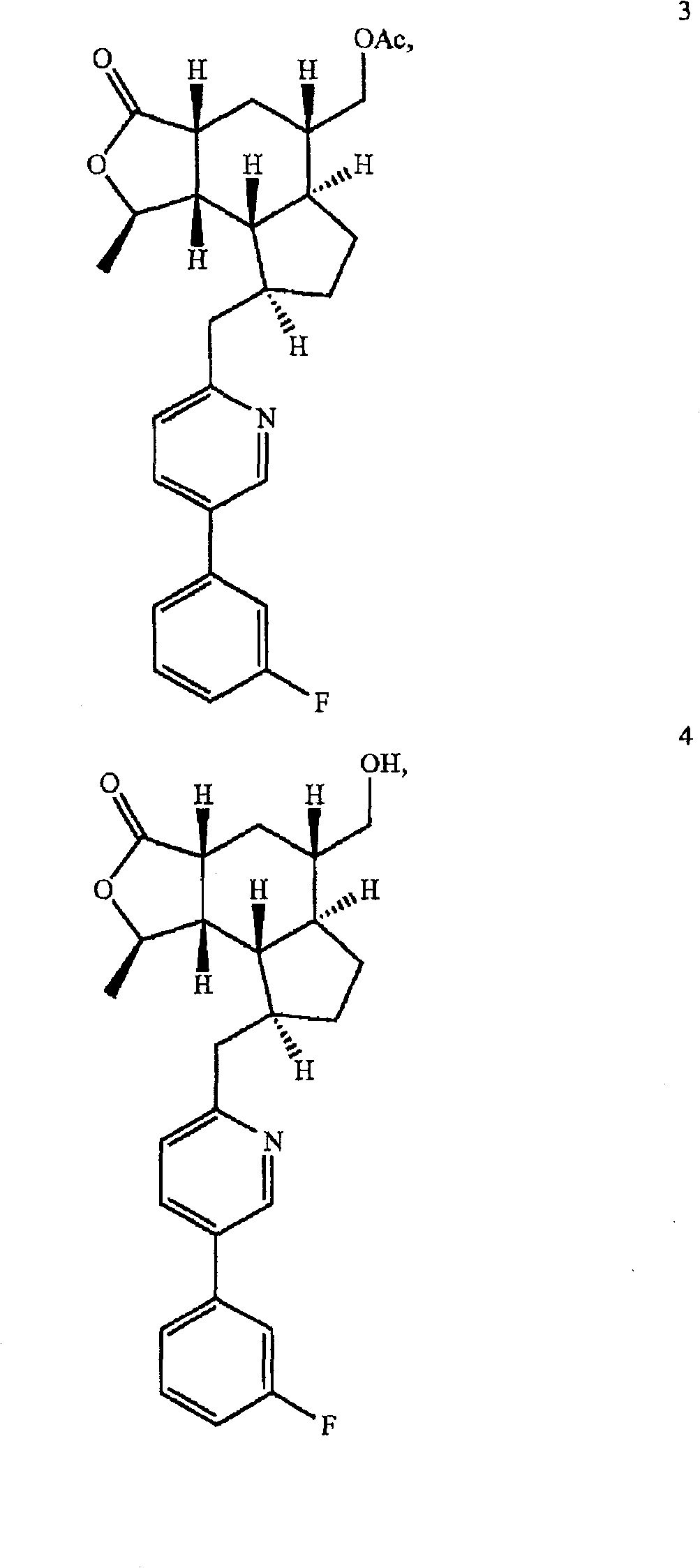
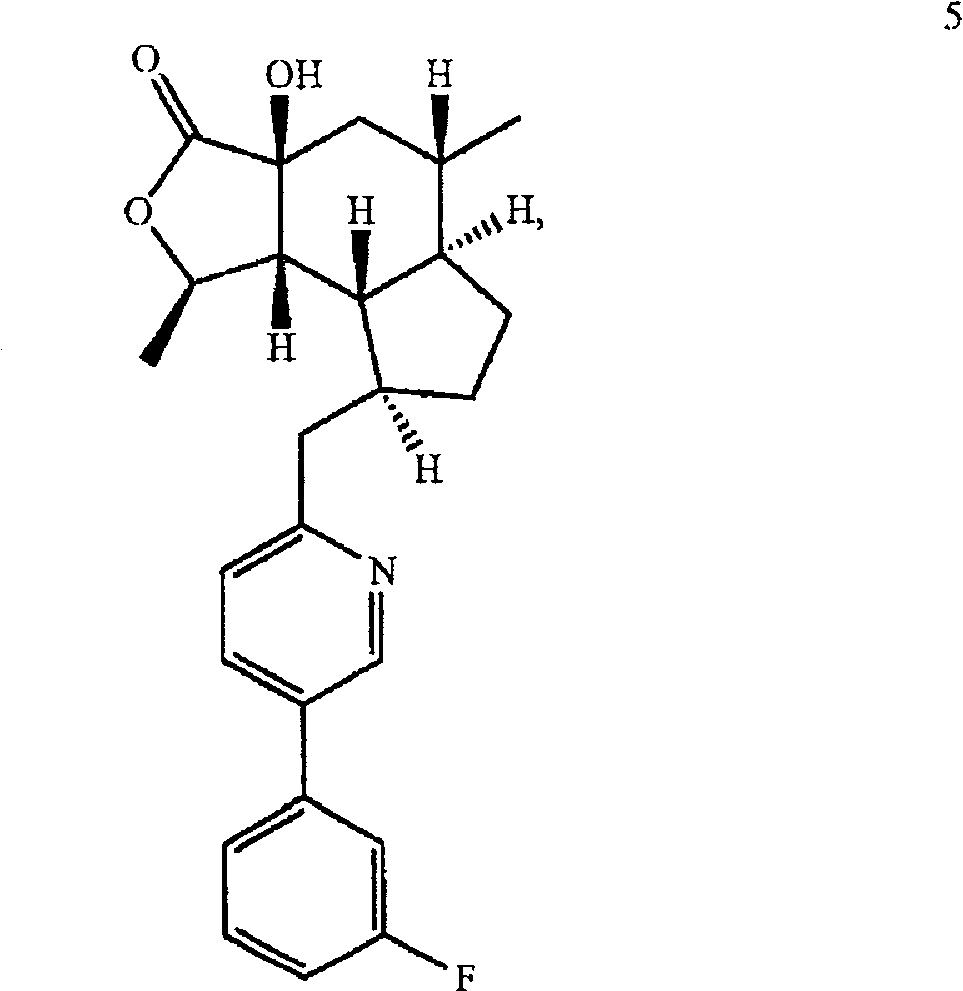
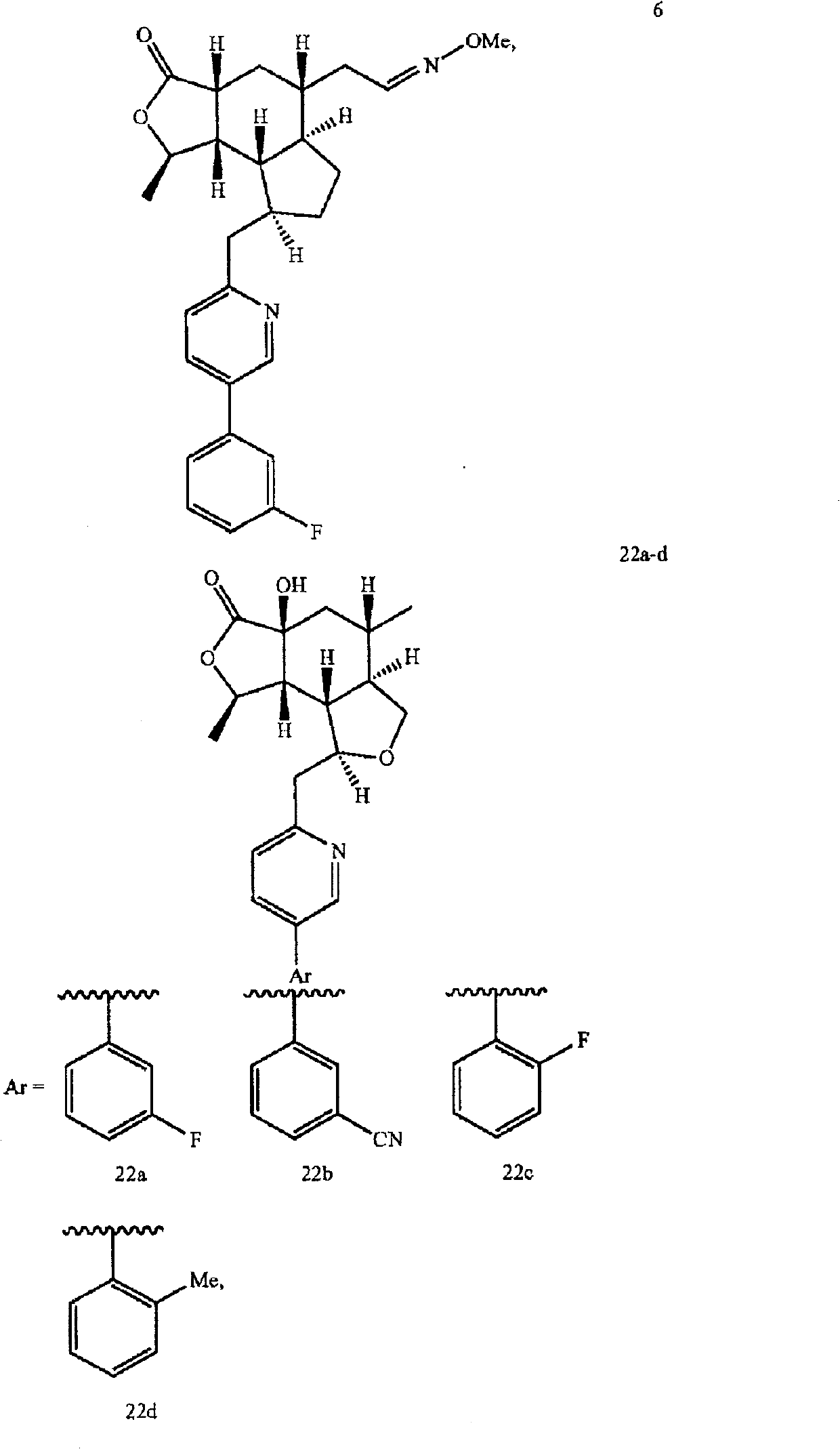
A compound represented by the structural formula and pharmaceutically acceptable salts and solvents thereof are disclosed, wherein: the single-dashed line between the ring carbons to which R<10> and R<34> are attached represents either a single bond or a double bond; the double-dashed line between X and the carbon to which Y is attached represents either a single bond or an absent bond; X is -O- or -NR<6>- when the double-dashed line represents a single bond; X is H, -OH or -NHR<20> when the double-dashed line represents an absent bond; and other parameters are as defined herein. Also disclosed are pharmaceutical compositions and combinations containing said compounds and their uses as thrombin receptor antagonists and binders to cannabinoid receptors.
Description
Background technique Various himbacine derivatives and pharmaceutical compositions containing these compounds have been disclosed in US Patent Nos. 6,063,847, 6,645,987 and 6,326,380 and US Application Nos. 10 / 271,715, 10 / 671,216 and 10 / 412,982. In the treatment of diseases related to thrombosis, arteriosclerosis, restenosis, hypertension, angina pectoris, arrhythmia, heart failure, cerebral ischemia, stroke, neurodegenerative diseases and cancer, these hibacin derivatives can be used as Thrombin receptor antagonist. Thrombin receptor antagonists are also known as protease-activated receptor-1 (PAR-1) antagonists. Many hebacin derivatives can also bind to cannabinoid receptors, and can be used to treat rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, diabetes, osteoporosis, renal ischemia, stroke, cerebral Ischemia, nephritis, inflammatory conditions of the lung and gastrointestinal tract, and respiratory diseases (such as reversible airway obstruction...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D405/08C07D493/04A61K31/443A61K31/4433A61P9/00A61P25/28A61P11/00C07D405/06
CPCC07D405/06C07D493/04A61P1/00A61P1/16A61P11/00A61P11/06A61P11/08A61P11/16A61P13/12A61P15/10A61P17/00A61P17/02A61P17/06A61P19/02A61P19/08A61P25/00A61P25/06A61P25/14A61P25/16A61P25/28A61P27/02A61P27/06A61P29/00A61P35/00A61P43/00A61P7/00A61P7/02A61P9/00A61P9/04A61P9/06A61P9/08A61P9/10A61P9/12A61P3/10
Inventor M·V·彻利亚S·查卡拉曼尼尔Y·夏M·C·克拉斯比W·J·格林李Y·王E·P·韦尔特里W·吴M·P·格拉齐亚诺T·科索格罗M·真达那
Owner MERCK & CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com