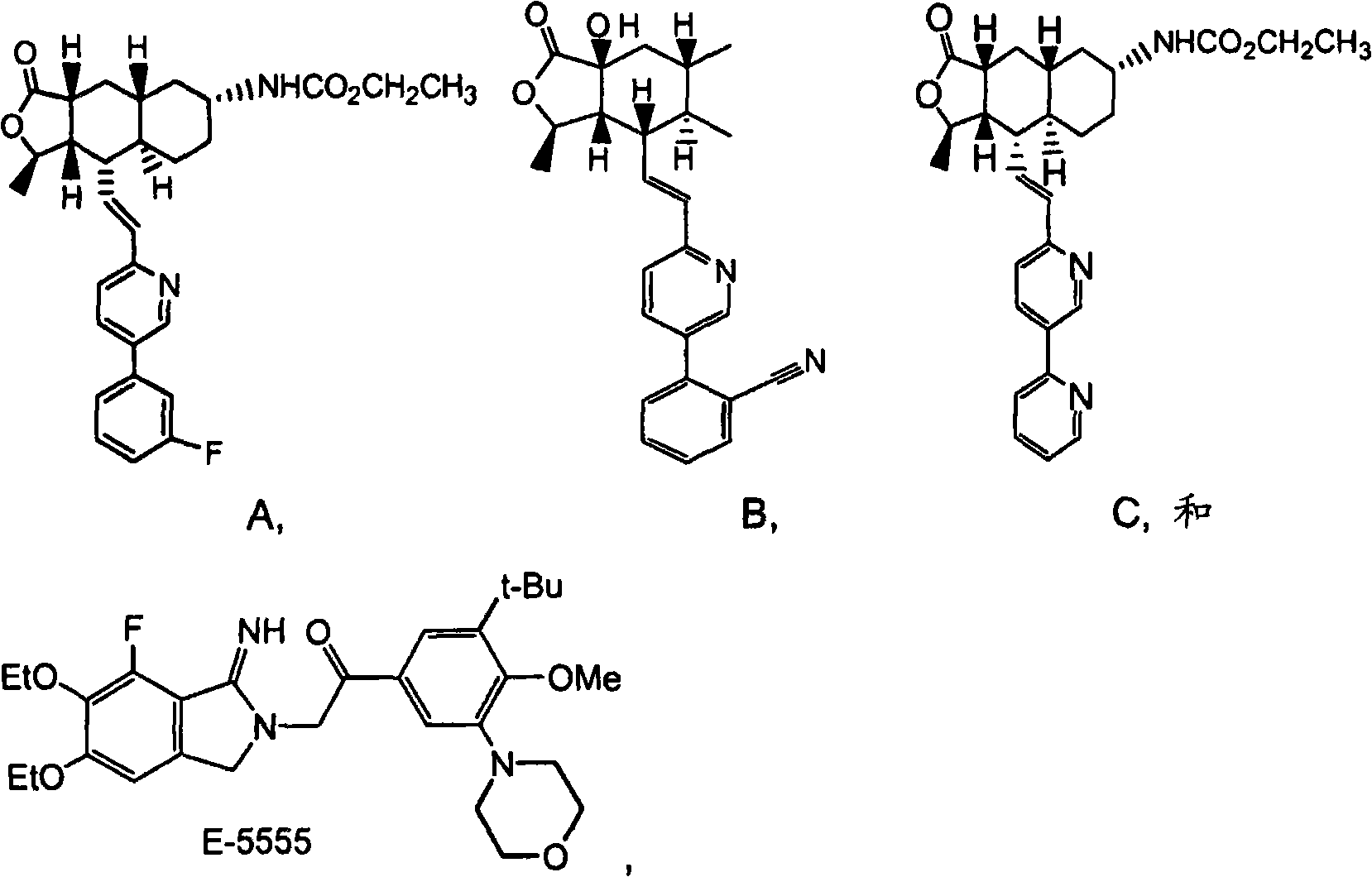
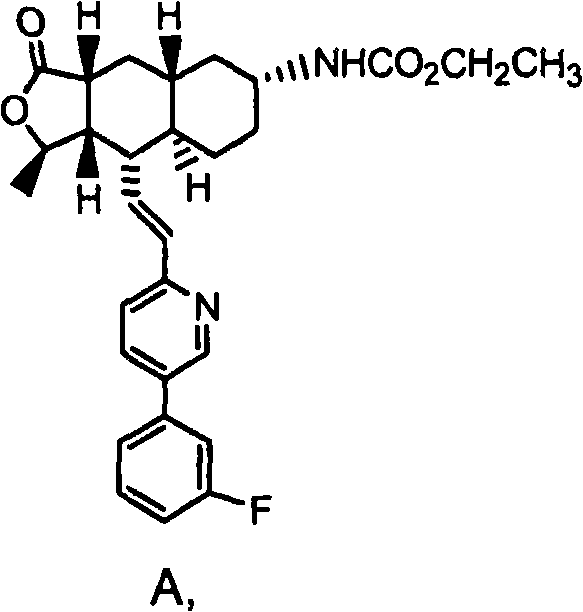
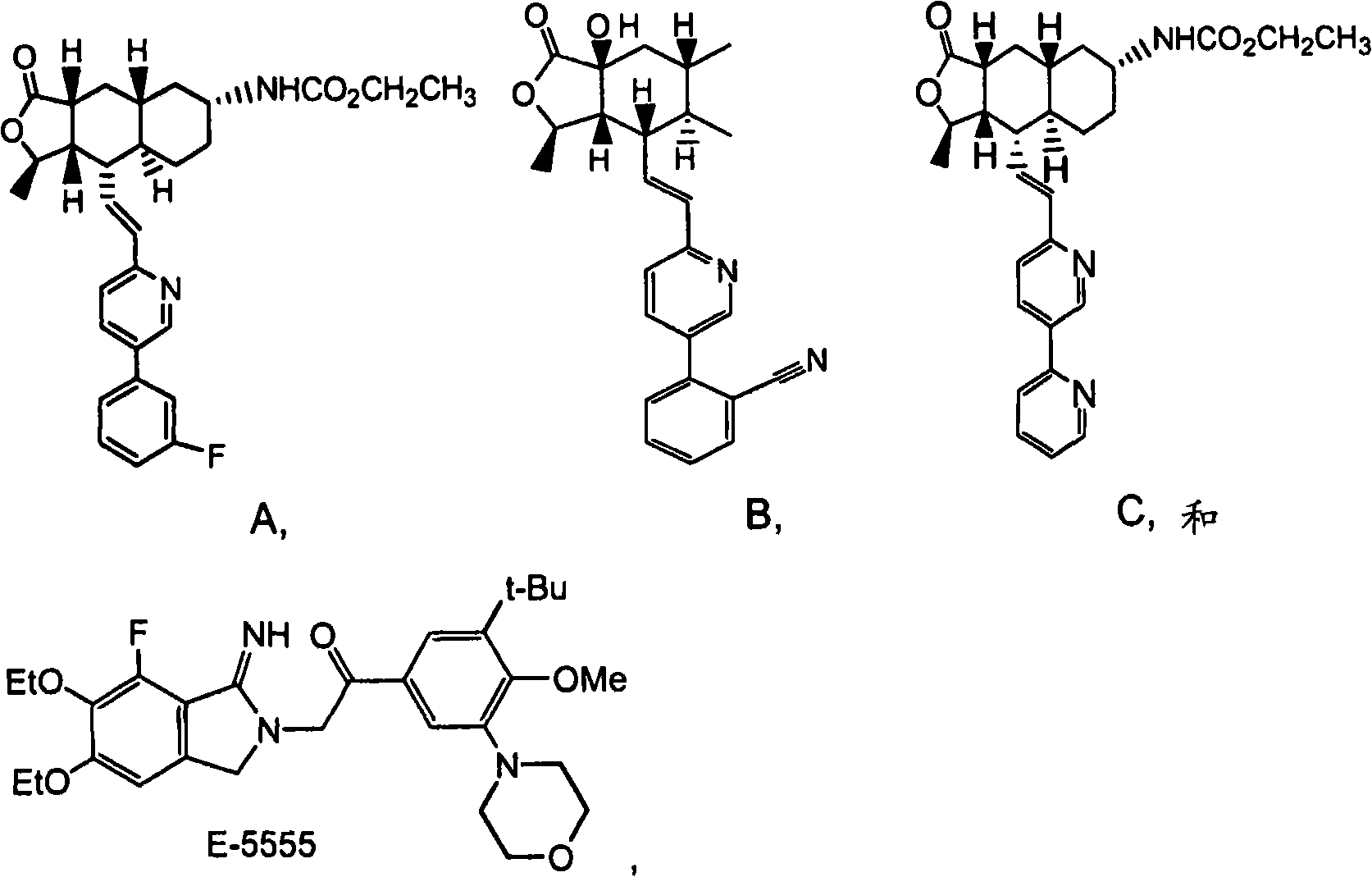
Rapidly disintegrating lyophilized oral formulations of a thrombin receptor antagonist
A thrombin receptor and antagonist technology, which is applied in the field of fast-disintegrating orally administered pharmaceutical compositions, and can solve the problem that patients cannot swallow solid preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 shows an example of a lyophilized formulation using sodium hydroxide as an excipient for pH adjustment.
[0056] Example 1
[0057]
[0058] The prototype of Example 1 showed a disintegration time of about 2 seconds with acceptable stability. When tested in an in vitro dissolution apparatus such as the apparatus described above, substantially 100% of the Compound A bisulfate salt dissolved within a time frame of 15 minutes.
[0059] buffer system
[0060] A prototype formulation was first prepared including NaOH as an excipient for pH adjustment. The use of NaOH is acceptable for initial pH adjustment of the suspension, however, the pH can then change over time due to dissociation of bisulfate into free base and counterion. This pH change can affect the properties of the final product. In order to stabilize the pH, it was recognized that a buffer system with appropriate buffering capacity is required. The purpose of the buffer system is to maintain th...
Embodiment 2
[0087]
[0088] Based on the foregoing, 40 mg loading dose lyophilized formulations of Compound A, or pharmaceutically acceptable salts and hydrates thereof, believed to be useful include those formulations comprising:
[0089] Gelatin in an amount of about 16 to about 19 mg, preferably about 17.5 mg;
[0090] Mannitol in an amount of about 14 to about 16 mg, preferably about 15 mg;
[0091] Sodium citrate in an amount of about 18 to about 19 mg, preferably about 18.7 mg; and
[0092] Citric acid, used in an amount of about 7 to about 8 mg, preferably about 7.7 mg.
[0093] Alternative embodiments in which the excipient components recited in Examples 1 and 2 above are replaced with other components within the same functional class are within the scope of the invention. Accordingly, embodiments wherein the gelatin is replaced with another polymer such as starch are encompassed by the invention. Similarly, embodiments wherein mannitol is replaced with another matrix formin...
example
[0100] table 3.
[0101] Element
Amt (mg)
Wt% **
Amt (mg)
Wt% **
5
23.2
10
37.6
Gelatin NF
8.985
41.7
8.985
33.8
Mannitol USP
7.188
33.3
7.188
27.1
Flavor mint 51296TP0551
0.150
0.7
0.150
0.6
Anhydrous Citric Acid USP
0.250
1.2
0.250
0.9
(---) *
(---) *
(---) *
(---) *
theoretical dry tablet
21.573
100%
26.573
100%
[0102] *Sublimated during lyophilization
[0103] **dry basis
[0104] acute coronary syndrome
[0105] The present invention also includes a method of treating a patient at risk of acute coronary syndrome by administering an effective amount of a rapidly disintegrating formulation of the aforementioned thrombin receptor antagonist. The term "effective amount" as used herein is understood to describe the amount of thrombi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com