Stimulation of cartilage growth with agonists of the non-proteolytically activated thrombin receptor
a non-proteolytically activated thrombin and cartilage growth technology, which is applied in the direction of prosthesis, peptide/protein ingredients, peptide sources, etc., can solve the problems of cartilage self-repair after injury, disease, surgery, etc., and the cost of cartilage repair from acute joint injury (meniscal lesions, patellar surface damage and chondromalacia) exceeds $1 billion annually. , to stimulate proliferation and matrix production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
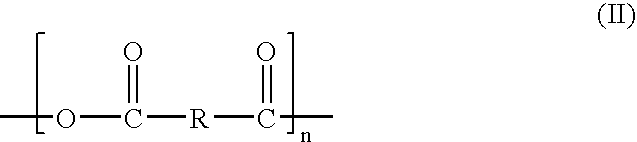
Image
Examples
example 1
Thrombin Binding to Rat Chondrocytes
[0046] Primary cultures of rat articular chondrocytes were isolated and prepared for in vitro analysis using established methods (see Kuettner, K E., et. al., J. Cell Biology 93: 743-750, 1982). Briefly, cartilage pieces were dissected from the shoulder of rats and the pieces were digested with trypsin for one hour and with collagenase for three hours in tissue culture medium (DMEM) at 37 C with stirring. The cells were plated in flasks at high density (50,000 cells / cm sq.) and were culture in DMEM containing antibiotics an ascorbic acid at 37° C. in an atmosphere of 5% CO2.
[0047] The specific binding of 125I thrombin to chondrocytes was carried out using established thrombin receptor binding assays as disclosed in U.S. Pat. No. 5,352,664 and Carney, D. H. and Cunningham, D. D., Cell 15:1341-1349 (1978). Briefly, highly purified human thrombin was iodinated and added to cultures of chondrocytes with or without unlabeled thrombin to correct for n...
example 2a
NPAR Agonist Stimulation of Bovine Chondrocyte Proliferation
[0049] Primary cultures of bovine chondrocytes were prepared using the procedure described for rat chondrocytes in Example 1. The cultures were subcultured into 24 well plastic dishes at a low density and placed in 1% serum. Addition of the NPAR agonist TP508 to these cultures at concentrations of 1.0 or 10 μg / ml by itself did not stimulate cell proliferation. In contrast, addition of these concentrations of TP508 together with a small amount of thrombin co-mitogen, resulted in a small, but significant (p<0.05) increase in cell number relative to that seen in thrombin alone after three days in culture.
example 2b
NPAR Agonist Stimulation of Bovine Chondrocyte Proteoglycan Synthesis
[0050] To determine the effect of NPAR agonists on proteoglycan synthesis, bovine chondrocytes were seeded into 96 well plates at a density of 2×105 cells per well and cultured in DMEM with 10% fetal calf serum. After establishment of these multi-layer cultures, the medium was replaced daily with DMEM containing 1% serum with indicated concentrations of TP508 from 1 to 100 μg per ml (Table 1). After 6 days in culture with daily changes of culture medium with or without TP508, 35S sulfate was added to the medium and incubation continued for an additonal 24 hours. As shown in Table 1, treatment with high concentrations of TP508 (100 μg per ml) increased 35S sulfate incorporation relative to untreated cells by more than 10-fold.
TABLE 1Effect of the NPAR agonist TP508 on 35S sulfate incorporation inbovine chondrocyte cultures.Mean CPMTreatment1% SerumStd. Dev of MeanControl49753552TP508 (1 μg / ml)47012692TP508 (10 μg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Biocompatibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com