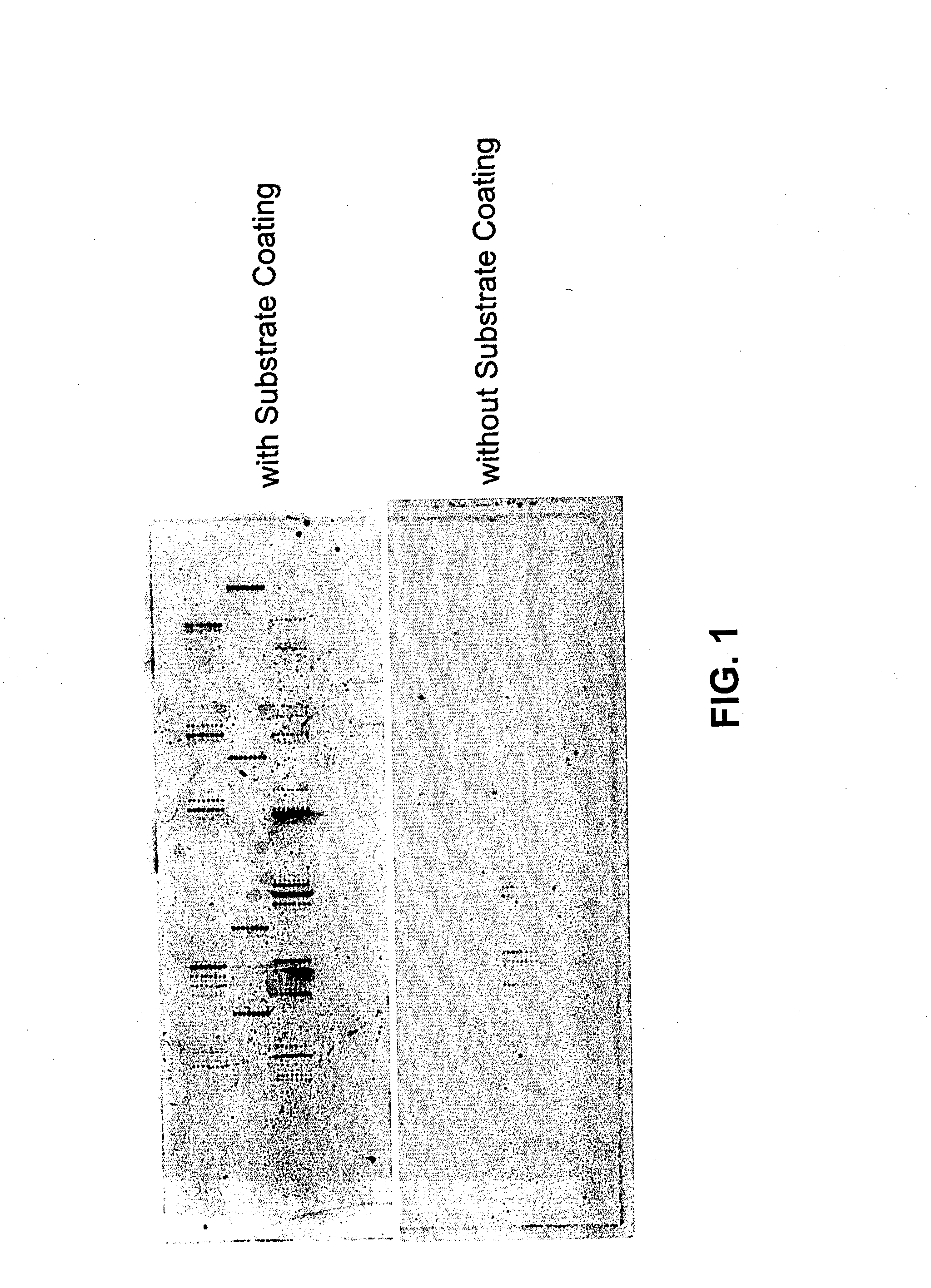
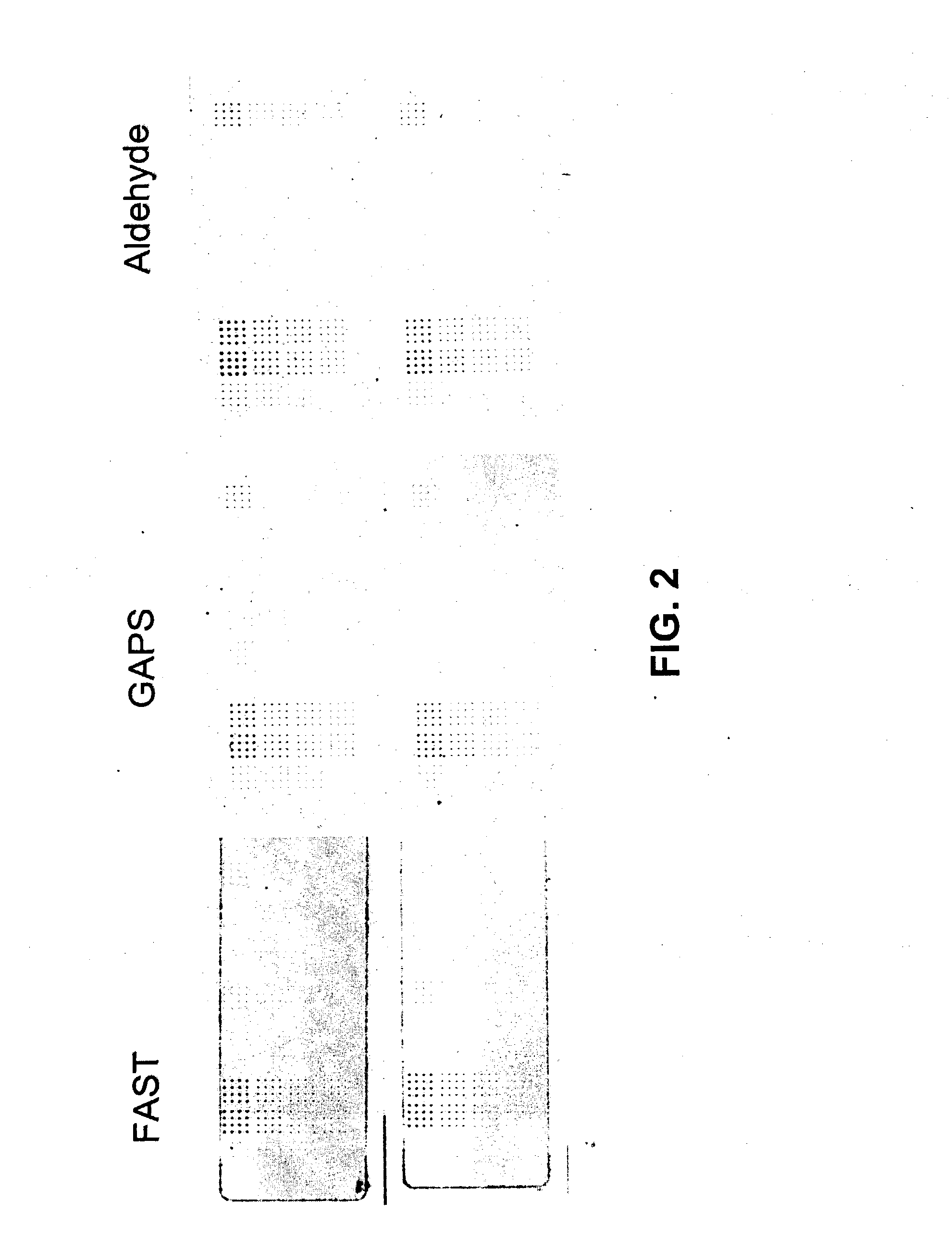
Methods for conducting assays for enzyme activity on protein microarrays
a technology of enzyme activity and microarrays, applied in the field of methods of conducting enzyme activity assays on microarrays, can solve the problems of time-consuming process, insufficient correlation of transcriptional profiles with cellular protein levels or protein activities, and detailed analysis of individual protein biochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
6.1. Example I
Kinase Activity Assay on Microarray Materials & Reagents
[0283]
3 Materials / Equipment / Reagents Vendor Part Number Disposables / Reagents Gamma-AT.sup.33P (10 .mu.Ci / .mu.l, Perkin Elmer NEG602H250UC 250 .mu.Ci) Histone, Calf Thymus Calbiochem 38205 Casein Sigma C-4032 Myelin Basic Protein Sigma M-1891 Poly-glutamic acid-tyrosine Sigma P-2075 PBS Tablets American AB11108 Bioanalytical Tween-20 American AB02038 Bioanalytical 60 .times. 24 mm Hybridization Cover Schleicher & 10 484 907 slips Schuell Equipment Cyclone Phospho-imager Perkin Elmer B431220 8 .times. 10 Autoradiography Cassettes Fisher FB-XC-810 Phosphor Storage Screens (MS) Perkin Elmer 7001723 Lab Rotator Lab-Line 1314 Instruments Eppendorf Centrifuge (5810) Fisher Scientific 05-400-60
[0284] Reagent / Stock Preparation
[0285] Kinase Substrate Stocks
[0286] Dissolve protein substrates in 20 mM Tris to a final concentration of 10 mg / mL.
[0287] 1 L of 1.times.PBS
[0288] Dissolve 5 PBS tablets in 1 L dH2O.
[0289] Mix thorou...
example ii
6.2. Example II
Inhibitor Specificity Profiling
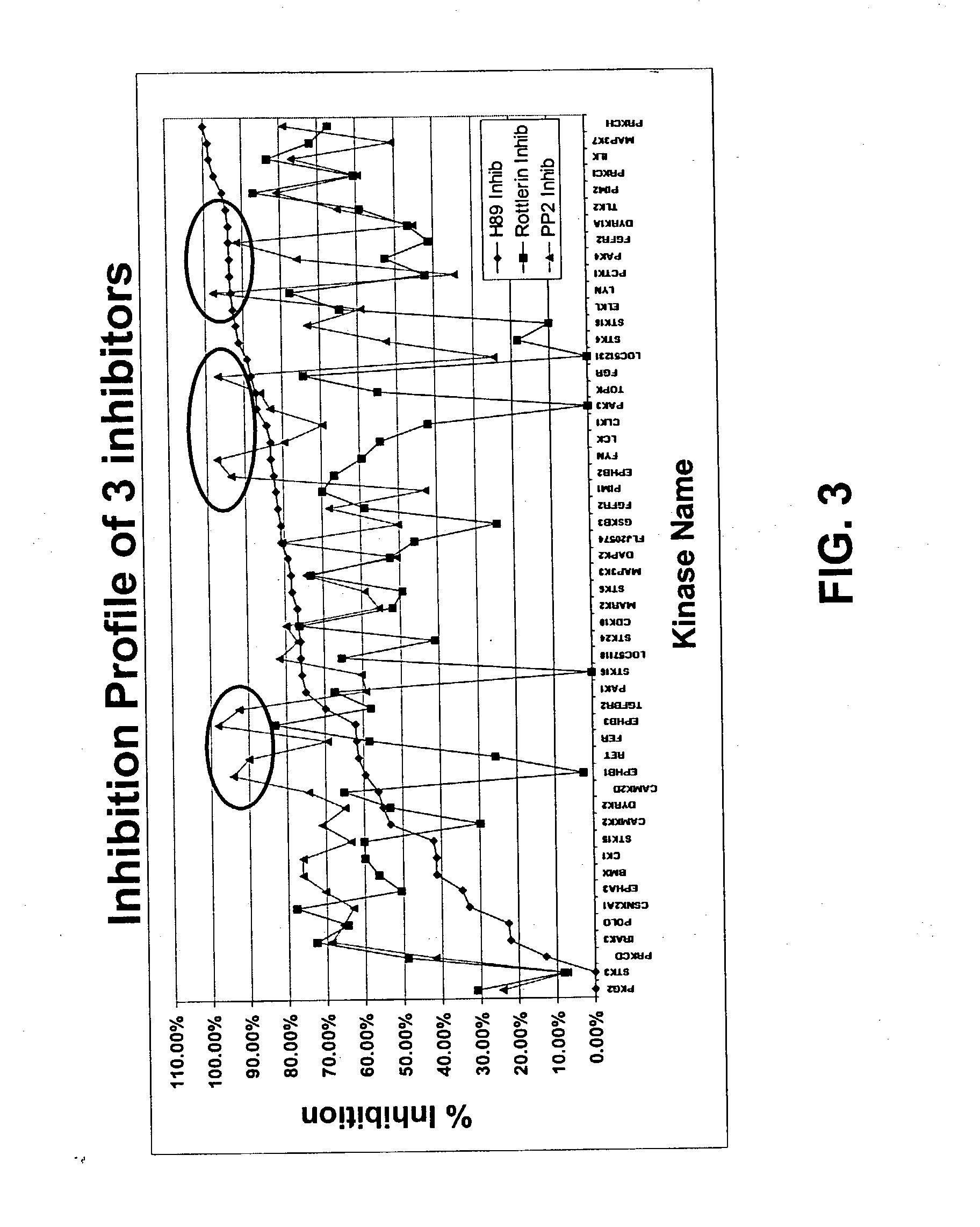
[0315] Fifty different kinases were immobilized on a slide together with a substrate as described in section 6.1. A mixture of Myelin Basic Protein (MBP), histone and casein was used as substrate. The kinase reactions were performed in the presence of H89 inhibitor, Rottlerin inhibitor or PP2 inhibitor (FIG. 3). The inhibitors were obtained from Calbiochem. The PP2 inhibitor is an inhibitor of tyrosine kinases. The concentration of inhibitor was 100 .mu.m for each inhibitor. The control reaction was performed in the absence of inhibitor. The specificity of the assay is demonstrated by the fact that PP2 inhibitor strongly inhibits tyrosine kinases (see circled data points in FIG. 3).
example iii
6.3. Example III
Dose-Response Analyses
[0316] Microarrays were prepared with 10 wells / slide, wherein the kinases EPHB3, FYN, and PRKCD and their substrate were immobilized in each well. The slide was coated with substrate essentially as described in section 6.1. Subsequently, a gasket with 10 openings was applied to the surface of the slide thereby creating 10 wells, i.e., the gasket provides the barriers between the wells. The accession numbers for the different kinases in the NCBI database are: for FYN: NM.sub.--002037; for PRKCD: NM.sub.--006254; and for EPHB3: NM.sub.--004443. A mixture of Myelin Basic Protein (MBP), histone and casein was used as substrate. The kinase reaction was performed in each well with a different concentration of PP2 inhibitor. The dose-response curve is shown in FIG. 4a. The data show that PP2 strongly inhibits the tyrosine kinases FYN and EPHB3 but not the serine / threonine kinase PRKCD. In a second exeriment, the kinase reaction was performed in each we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com